BioByte 017
treating vision loss with optogenetics and BCI, what end to end biology looks like, building a science startup with little funding, raising the bar in generative molecular design, and more!
Welcome to Decoding Bio, a writing collective focused on the latest scientific advancements, news, and people building at the intersection of tech x bio. If you’d like to connect or collaborate, please shoot us a note here. Happy decoding!
Happy Tuesday! Hope everyone had a warm start to the week (special shoutout to our friends in Boston for making it through the brutal cold this weekend).
This week brings more “generative AI in bio” content as well as some exciting new research in neuro, including breaking down Max Hodak and Eric Knudsen’s new paper on what their BCI company Science is up to. Let’s get into it.
What we read
Blogs
Great science companies can start with little funding [Alex Schubert, 2023]
In this brief piece, Alex Schubert reminds us of the power of limited resources in building bio companies. Though large venture financings can serve as catalysts for driving non-linear transformation in bio, it’s not the only way to finance innovation in life sciences.
For example, Regeneron was started in 1988 with $1M (around $2.5M in today’s money) and Genentech in 1976 with $100k (would be around $500k now). In a separate post, Alex summarizes some of the most impressive bio founding stories here.
Generative Molecular Design - We Need to Raise the Bar [Pat Walters, 2022]
Why it matters: Generative molecular design has largely focused on generating molecules with strong binding affinity to a given target, but has mostly ignored the important question of selectivity and off-target effects. Generative AI for molecular design should prioritize selectivity in addition to binding affinity in order to improve side profiles and therapeutic utility of potential drugs. Generative molecular design has grown rapidly over the last several years. The field has largely focused on a single objective function: generating molecules optimized for binding affinity to a given target. For example, many papers have applied generative algorithms to the design of kinase inhibitors. Kinases are important targets in many therapeutic areas, and there are 70+ FDA-approved drugs that target kinases. The problem for generative AI is that kinases perform many functions in the cell, and off-target effects of a kinase inhibitor can cause serious side effects. Therefore, generating a kinase inhibitor that is specific for the kinase of therapeutic interest is critical, yet the field of generative molecular design has largely ignored the question of kinase selectivity.
Notes on End to End Biology [Nintil, 2023]
José poses the important questions of “is this time different” and “what does true end to end biology look like” in his recent essay on End to End Biology. It’s been a decade since AI-first drug discovery companies like Recursion and Exscientia promised us data on one end and predictive output on the other end. But naysayers like to come back to the one looming point that there hasn’t yet been an approved AI-derived drug. With new advances in generative output Jose investigates that question of “is this time different” concluding that we need to recognize how little we know about biology and if biology can’t be fully understood then we need to instead prioritize “gathering everything we learn into first-class quality databases that might enable computers to make sense of what we have learned”. He goes on to discuss the implications of AlphaFold, relates drug discovery to self-driving cars, and touches on how we can’t simply train an LLM on science because so little is known about the human body.
“Progress in basic biology research tools has created the potential for accelerating medical progress; however, this potential will not be realized unless we fundamentally rethink our approach to biomedical research. Doing so will require discarding the reductionist, human-legibility-centric research ethos underlying current biomedical research, which has generated the remarkable basic biology progress we have seen, in favor of a purely control-centric ethos based on machine learning. Equipped with this new research ethos, we will realize that control of biological systems obeys empirical scaling laws and is bottlenecked by biocompute. These insights point the way toward accelerating biomedical progress.”
What ChatGPT and generative AI mean for science [Stokel Walker, Van Norden, Nature 2023]
How will large language models transform science? Each week over the last few months, we’ve detailed stories of companies applying large models to proteins, images, clinical data, and more. This piece explores a number of considerations for how generative AI will impact science as well as the related ethical and safety concerns. This past week, we were blown away by generative AI’s manuscript editing capabilities:
Postdoc survey confirms widespread dissatisfaction among US researchers. [Laurie Udesky, Nature, 2023]
Not a new theme on the blog, a recent survey conducted by the US National Postdoctoral Association found that 94.8% (read: nearly all) of the survey’s respondents claim their low-pay negatively affects their personal and professional lives—putting numbers to a growing sentiment we have all heard. In addition to low pay, the postdocs surveyed, many of whom have families to support, also cite low job security, career uncertainty, and ever decreasing funding for their roles a contributor to growing dissatisfaction.
Some open questions—how do we better support those contributing to our understanding of science? What are some alternatives to postdoctoral research positions for those continuing in academia and otherwise? How do we reimagine this model?
Academic papers
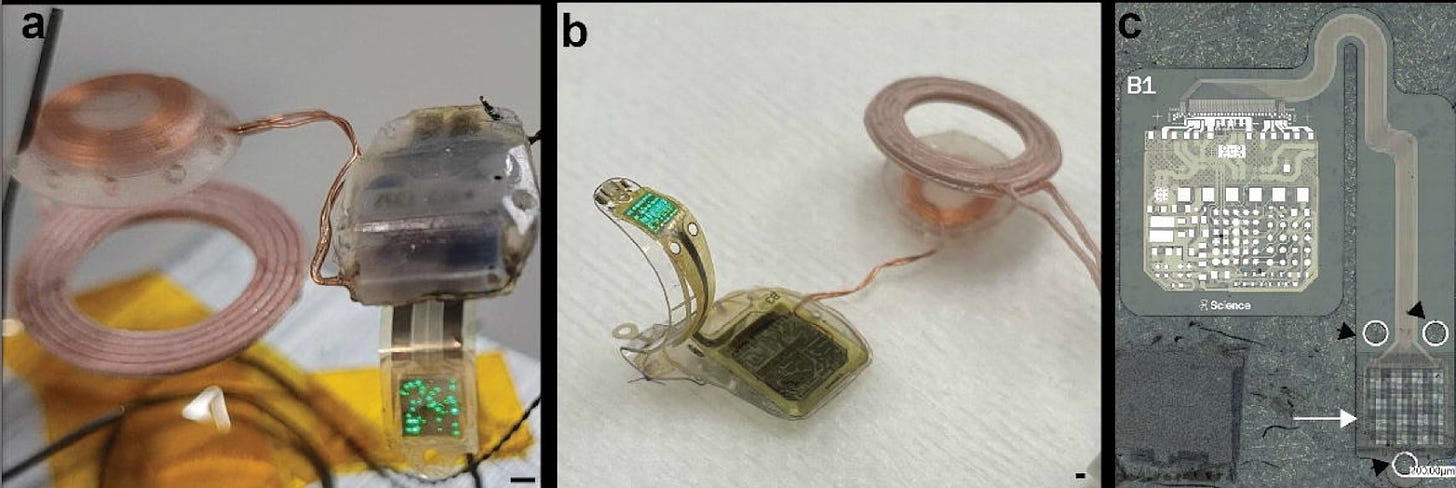
A thin-film optogenetic visual prosthesis [Knudsen et al., BioRxiv, 2022]
Why it matters: A new brain-computer interface (BCI) company called Science published their first paper describing a optogenetic-based BCI system for the treatment of photoreceptor pathologies that cause vision loss such as macular degneration. The data provide a proof of concept that an LED-based retinal prosthesis combined with opsins delivered by AAV to retinal ganglion cells can drive activity in the primary visual cortex in a rabbit model of photoreceptor degeneration. Max Hodak’s BCI company Science published their first pre-print on a BCI system for the treatment of retinitis pigmentosa and macular degeneration. The device is a wireless, thin-film LED implant for the optogenetic stimulation of photoreceptors in the eye called retinal ganglion cells (RGCs). RGCs were genetically modified to express opsins, light-sensitive ion channels that can be activated (causing neurons to change their activity) when exposed to light delivered by LEDs. Opsins were delivered using AAV vectors engineered to access the retina.
Optogenetic stimulation of the retina using the device elicited activity in the primary visual cortex. Because animals were anesthetized, we don’t yet know if visual perception was restored. Further work in awake behaving animals, such as while performing a visual discrimination task, is needed. The company will also have to sort out the challenging issue of coding schemes in RGC subtypes. There are different subtypes of RGCs (ON and OFF cells) that increase or decrease their firing rates in response to optogenetic stimulation. In order to restore visual perception, Science’s prosthesis will need to incorporate information about this neural code to guide stimulation at cellular resolution.
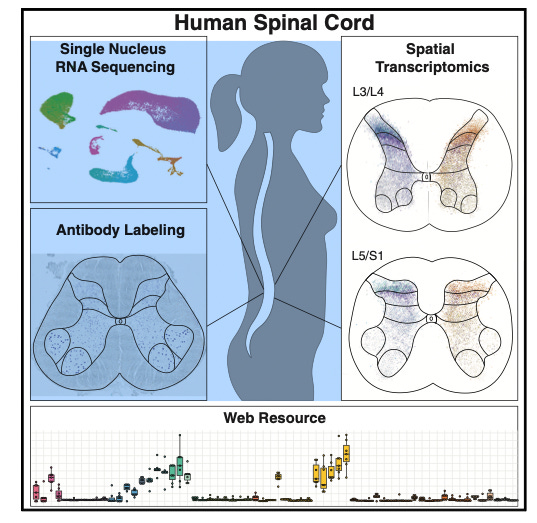
A cellular taxonomy of the adult human spinal cord. [Yadav et al., Neuron, 2023]
Why it matters: the authors paint a more comprehensive picture of one of the most important structures in our body that is involved in sensory processing, autonomic control, and movement. This provides information that can be used in the development of new therapies—both those that involve degeneration of certain neuronal and glial clusters and those that involve loss of cell-signaling and coordination.Advances in spatial transcriptomics, single-nucleus RNA sequencing, and immunohistochemistry come together to form a map of the cell types that make up the adult human spinal cord. The researchers isolated nuclei from donor lumbar spinal cord tissue and distinguished cell classes from each other (neurons, astrocytes, microglia, oligodendrocytes, etc). They also analyzed spinal motoneurons which are known to undergo modifications with neurodegenerative disease. They found “that compared with other spinal neurons, human motoneurons are defined by genes related to cell size, cytoskeletal structure, and ALS, suggesting a specialized molecular repertoire underlying their selective vulnerability.” Looking at cellular taxonomy as a whole for the spinal cord gives us more insight into cell signaling and motor control, with implications ranging from chronic disease to neurodegenerative disease.
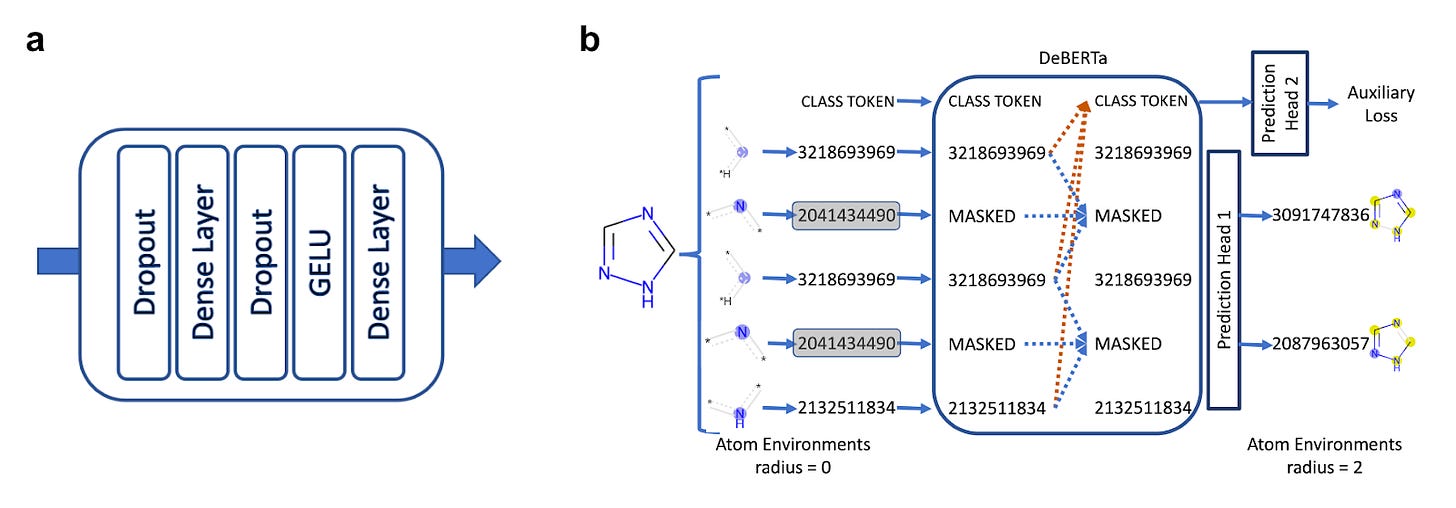
MolE: a molecular foundation model for drug discovery [Mendez-Lucio et al., arXiv, 2022]
Why it matters: Machine learning has been used in chemistry for decades, yet representing a molecule in a way that is compatible with machine learning algorithms and that also minimizes information loss remains a challenge. MolE uses an adapted transformer model that can use graphs as inputs and create tokens that contain information of the atom itself and those around it (their environment). Given the increased amount of atomic environment information per molecule, MolE was able to achieve state-of-the-art results on 9 out of 22 ADMET tasks from the Therapeutic Data Commons.The team at Recursion reported on MolE, a transformer model adapted from the existing model architecture DeBERTa. This model can learn molecular embeddings directly from molecular graphs, which contains information of each atom and it environment (with radius = 2, i.e. “all atoms and their associated bonds that are separated from the masked atom by two or less bonds”). The authors believe this approach can help solve problems with classical molecular fingerprints such atomic ‘collisions’ when using bit vectors.
What we listened to
Immunometabolism—Josh Elkington chats with UCSF postdoc Greg Timblin about immunometabolism, inflammation, and chemistry. This is a great discussion for those interested in lesser known areas of immunology.
Recursion Download Day—Recursion hosted their first download day for investors a few weeks ago in Salt Lake City. This is a cool inside look at the company’s R&D strategy, pipeline, and partnerships.
Some of our favorite investors take to Eric Newcomer’s podcast Dead Cat to chat about their new fund and why something monumental is happening in bio today.
In case you missed it
Homeworld Collective—a new nonprofit and community focused at the intersection of biology and climate.
What we liked on Twitter







Field Trip



Did we miss anything? Would you like to contribute to Decoding Bio by writing a guest post? Drop us a note here or chat with us on Twitter: @ameekapadia @ketanyerneni @morgancheatham @pablolubroth @patricksmalone