BioByte 019: broken bacterial antibiotics pipelines, mini-organs on the rise, LTR retrotransposons in gene delivery, psychedelics that promote neuroplasticity, and more
Welcome to Decoding Bio, a writing collective focused on the latest scientific advancements, news, and people building at the intersection of tech x bio. If you’d like to connect or collaborate, please shoot us a note here. Happy decoding!
Sorry we are a day late this week! You might be wondering why? We think we have a good reason… We were actually in a deep philosophical debate about whether it could ever be feasible to unfold a misfolded protein.
A budding biophysicist on our team shared: “The problem is that misfolded proteins - and particular types of aggregates - sit at the very bottom of an entropic trap. Energy requirements to unfold are almost impossible to achieve, making targeting for degradation is much more feasible.”
And from ChatGPT:
What do you think? Capping off another big week in bio. Happy Decoding!
What we read
Blogs
The Bacterial Antibiotics Pipeline: Still Broken, No Fix in Sight [Natalie Ma, 2023]
In a recent blog post, Natalie Ma touches on a very important issue—the lack of drug development happening in antibiotics. It’s long been known that the bacterial antibiotic market is far from favorable. Some studies quote that the payout for a successful antibiotic drug averages around $47M, which is nowhere near the amount necessary to justify a >$1B investment in creating that drug. For several years, pharmaceutical companies have been shifting focus away from antibiotic development to more economically favorable markets such as oncology and even rare disease. J&J just announced that they were exiting the antibiotics market, shutting down what appears to be rather underdeveloped programs, and laying off staff. They aren’t the first pharma outfit to make this switch in the last couple of years.
If profit is volume multiplied by price, the antibiotics market suffers from a lack of sufficient amounts of both. That being said, antibiotic resistance is a growing problem. One that shouldn’t be taken lightly given that the last truly original class of antibiotics was discovered in the 1980s and the number of resistant strains keeps increasing. We desperately need reform in the antibiotics market to meet what will become a dire need.
Introducing NEJM AI [New England Journal of Medicine, 2023]
This past week, the publisher of the New England Journal of Medicine (NEJM) announced the upcoming launch of NEJM AI, which will “identify and evaluate state-of-the-art applications of artificial intelligence to clinical medicine. NEJM AI will provide reviews, policy perspectives, and accessible educational material targeted at practicing physicians and clinician leaders interested in applying AI, computer scientists seeking to translate algorithmic advances to clinical practice, and policymakers and regulators.”
NEJM AI will be published online only once a month and will also include a bi-monthly newsletter and podcast called “NEJM AI Grand Rounds Podcast.” The list of editors is an impressive bunch including Dr. Isaac Kohane, Editor in Chief, as well as Dr. Daphne Koller, CEO of Insitro, and Dr. Krishna Yeshwant, Managing Partner at Google Ventures.
You can follow along at @NEJM_AI!
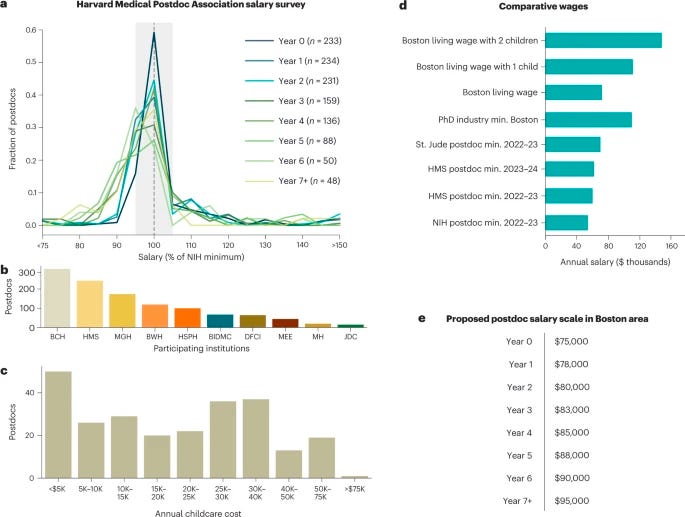
Retaining postdocs by recognizing their worth [Nature Biotechnology Career Feature, February 2023]
The biomedical postdoc shortage is becoming a problem, yet not one as challenging as the biology and chemistry that many postdocs grapple with. Despite the plentiful supply of biomedical PhD holders and interest in academia, many postdoc holders are considering alternate options in search of higher salaries and more robust benefits packages.
The data makes it clear:
“In a two-person employed household in Boston’s Suffolk County, the annual living wage for a single individual has been estimated at $71,869 before taxes, which rises to $111,259 with one child and $148,148 with two children. These wages include only necessities such as housing, healthcare, childcare, transportation, food and civil costs. Yet the NIH de facto postdoc stipend for someone starting a postdoc in 2022–2023 is $54,840 a year.”
So where are they going? Biotech, pharma, consulting, venture capital, technology, and device companies seem to be valuing the expertise of biomedical postdocs differently from academia, as the market salary for a graduating PhD in Boston ranges from $100-120k.
How much longer can academia continue to justify low postdoc salaries due to their status as “trainees” despite the immense value of their specialized skill sets in the private sector? We aren’t certain, but we think there’s value in letting the data tell the story:
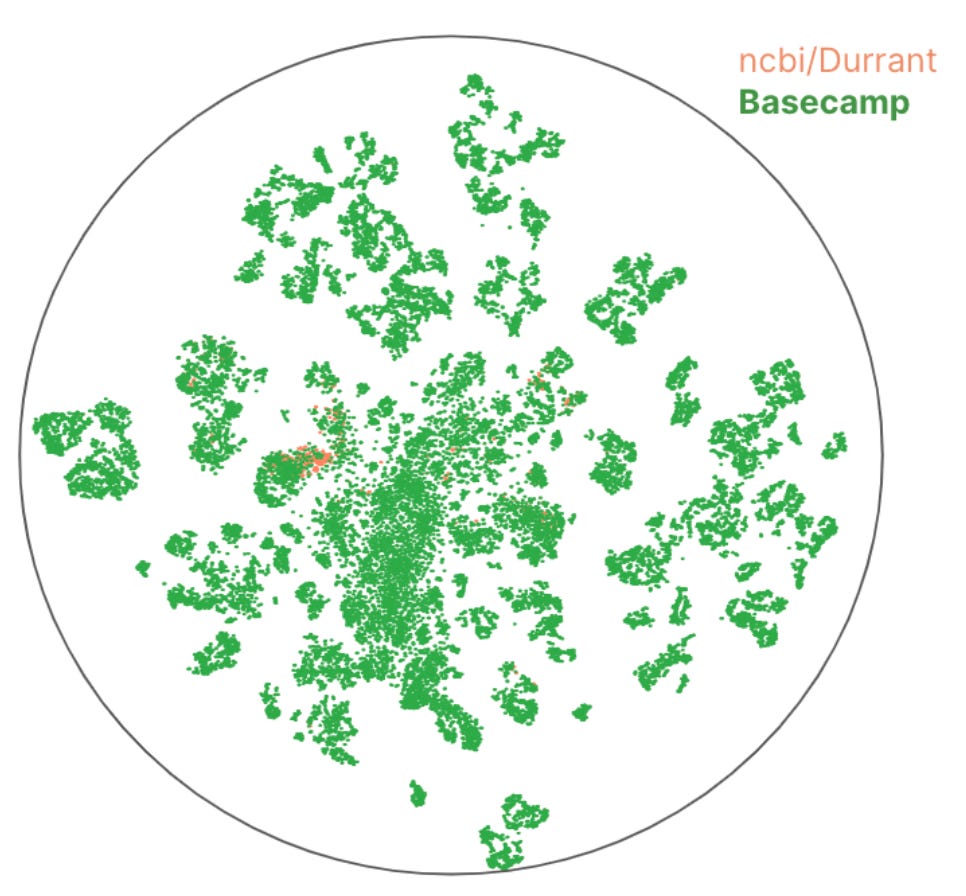
Expanding the repertoire of recombinases that can integrate large DNA fragments into the human genome by over 30X [Basecamp Research, 2023]
Nature has inspired countless tools in biotech companies, therapeutics to treat patients, and even some of the algorithms used in machine learning. One of the latest nature-inspired technologies is ‘gene writing’, which has become one of the most popular fields in biotech with companies such as Prime Medicine and Tessera leading the pack. Gene writing entails inserting large DNA strands into host genomes. There are several systems by which writing can be achieved, such as Large Serine Recombinases (LSRs). These are DNA integrases that allow the insertion of genetic elements in bacterial host genomes.
Using its own knowledge graph representing its ‘omic database which was gathered from 70 expeditions around the world, the company Basecamp Research was able to discover ~34K LSR clusters. This is about 30x more LSR clusters than the NCBI genomic database mining study.
Whilst the discovery of novel LSR sequences is important, identification of the integration site is even more important, as it means that one can map each LSR to where it will insert the payload DNA in the genome.
Mini-organs attract big pharma [Asher Mullard, Nature Reviews Drug Discovery, 2023]
Nature Reviews Drug Discovery published an interview with Hans Clevers, Head of Pharma Research and Early Development at Roche on the role of organoids in drug discovery. Some key takeaways:
Clevers believes organoids will play a role in every stage of drug development, from target ID and validation to safety and efficacy testing, to biomarker identification and patient stratification in clinical trials. The key roadblock is the validation of organoids as accurate model systems to sufficiently high standards to satisfy biopharma and regulatory bodies.
There have been a few examples where the majority of the data in an IND filing have come from organoids. This is likely to become more common with the FDA modernization act.
Organoids will eventually replace cell lines because they deliver more information while enabling experimental control and standardization. High-throughput screening is more difficult, however, because organoids grow in 3D and are harder to handle.
An important limitation of organoids is the lack of an immune system and other supporting structures like blood vessels. Efforts to build elements of the immune system into organoids will be needed to improve the clinical translatability of these models.
Immune Engineering [Elliot Hershberg, Century of Biology, 2023]
We’re big Elliot fans here at DB. In his latest essay, Elliot breaks down recent advances and promises in immune engineering, giving just enough context on the immune system for it all to make sense. He specifically talks about how increased investment in synthesis and sequencing capabilities underlie our ability to unlock more insight into the immune system. Using the classic example of CAR-T cells, we need to be able to sequence, edit, and synthesize long pieces of DNA for scalable CAR-T therapies. If you’re looking for more details on engineered immune cells themselves, read Eric Topol’s overview before reading this piece.
“This is why the intersection of the 4-S stack and immune engineering is so promising. Our DNA technologies are exponentially improving. Immune engineering is a tremendously high-value and high-impact application of genetic engineering and synthetic biology. We should get another level of compounding progress as reusable genetic circuits and components are developed over time.”
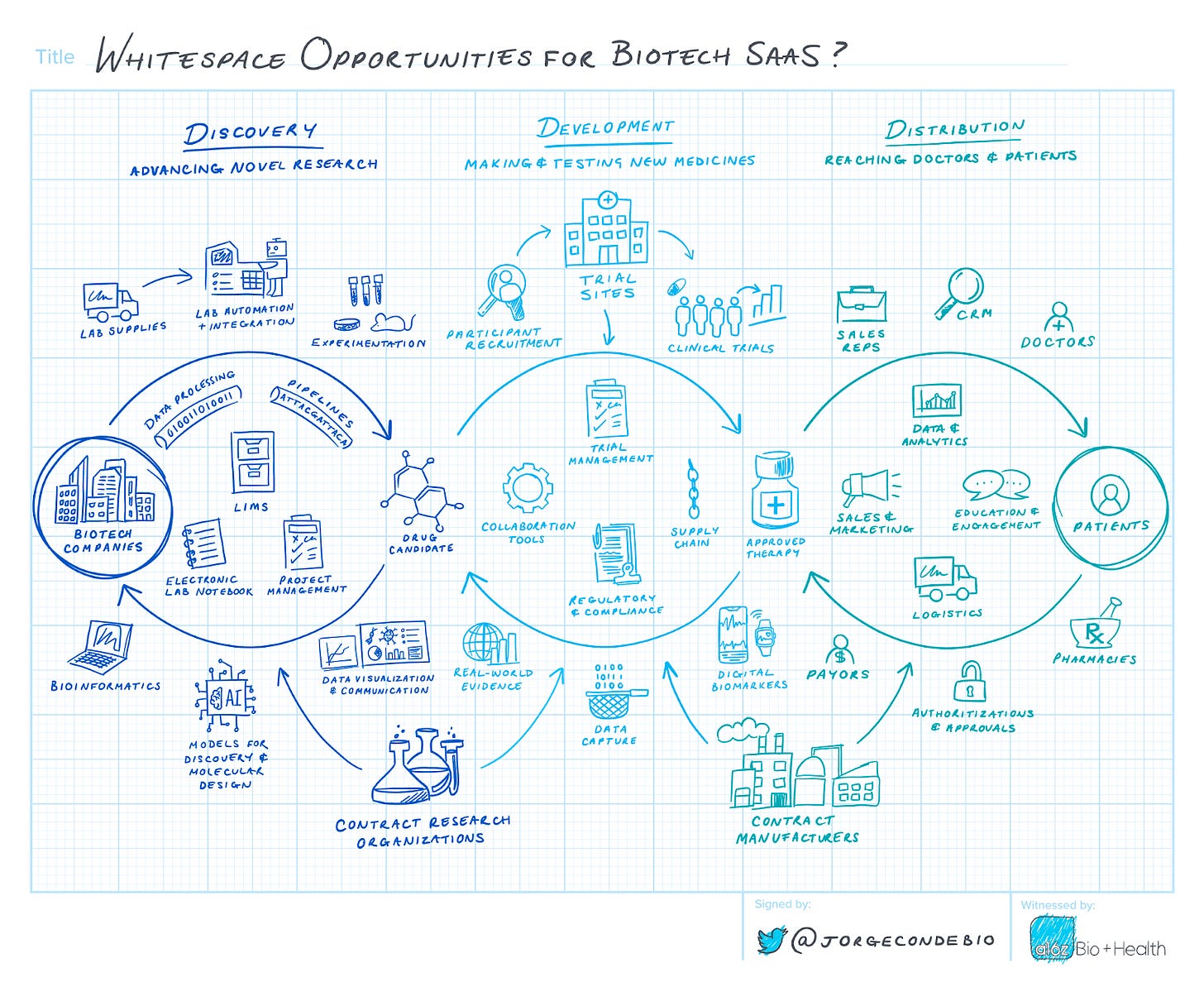
Doing More with Moore: Biotech’s Tech Moment [a16z, Jorge Conde and Jay Rughani]
Will software eat biology or will biology eat software? In this recent piece, our friends at a16z explore “whitespace opportunities for biotech SaaS” across discovery, development, and distribution.
Academic papers
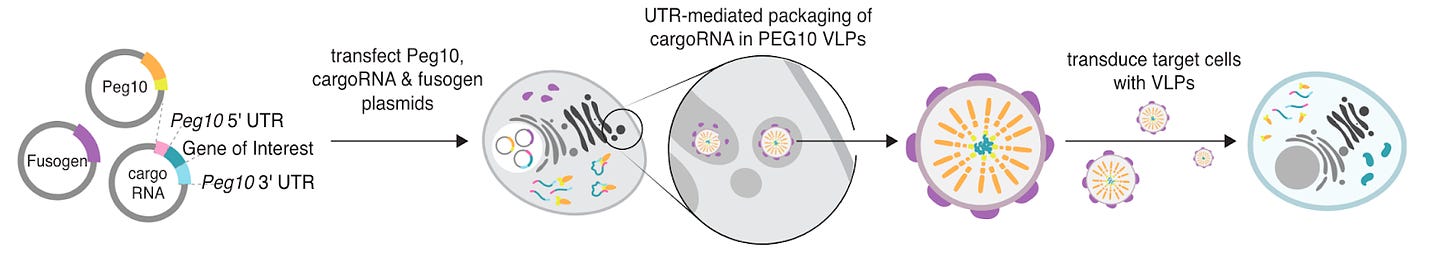
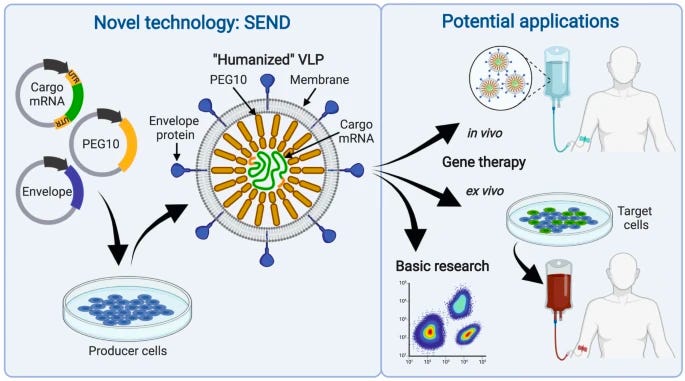
Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery [Segel et al., Science, 2021]
Why it matters: delivery of gene therapies is still a major development barrier for curing genetic disease. If our toolset included an easy-to-manufacture delivery method that could deliver any gene therapy (such as DNA, mRNA, gene editing…) in a targeted, low immunogenic, high-efficiency manner, we’d be able to cure many more diseases than we can today. Current approaches such as viral vectors (AAV, LVs) or non-viral vectors (LNPs, Exosomes, EVs) suffer from one or more of the above challenges which make them unsuitable as a main delivery method for gene therapy.Aera, a new gene therapy delivery company out of the Feng Zhang lab, launched with ~$200m in funding from Arch, GV, and Lux. Here we outline the findings of the original paper on which the company based its technology on.
More than 8% of our genomes is composed of sequences from long terminal repeat (LTR) retroelements, including retroviruses, that have integrated into our genome through evolution.
LTR retrotransposons are genetic elements that copy and paste themselves into different parts of the genome via an RNA intermediate. These retrotransposons have two genes: gag and pol.
The gag gene products associate to create virus-like particles. The pol gene encodes for the reverse transcriptase, integrase, and ribonuclease which reverse transcribes the copy RNA back into DNA, inserts the DNA back into the genome, and cleaves phosphodiester bonds between RNA nucleotides respectively.
The Zhang lab carried out a computational survey of mammalian capsid-forming gag homologs where they identified 48 gag-derived genes in the human genome and 102 gag homologs in the mouse genome, with 19 conserved between both of them. Those containing core capsid (CA) domains were further investigated. They then observed that some of these homologs assembled into capsids within extracellular vesicles released by the screened cells, with MmPEG10 being the most abundant. Therefore, they ascertained that PEG10 can bind its own mRNA and release it in the form of a virus-like particle.
The authors then showed that other mRNA payloads flanked by PEG10 UTRs could also be packaged. They added fusogenic vesicular stomatitis virus envelope protein (VSVg) in the producer cells so the payloads could be transferred to other cells. This system was named SEND (selective endogenous encapsidation for cellular delivery).
Given the targeting capabilities, the low immunogenicity due its derivation from humans, and its self-encapsulating abilities, it is possible SEND will become a leading competitor to LNP technologies.
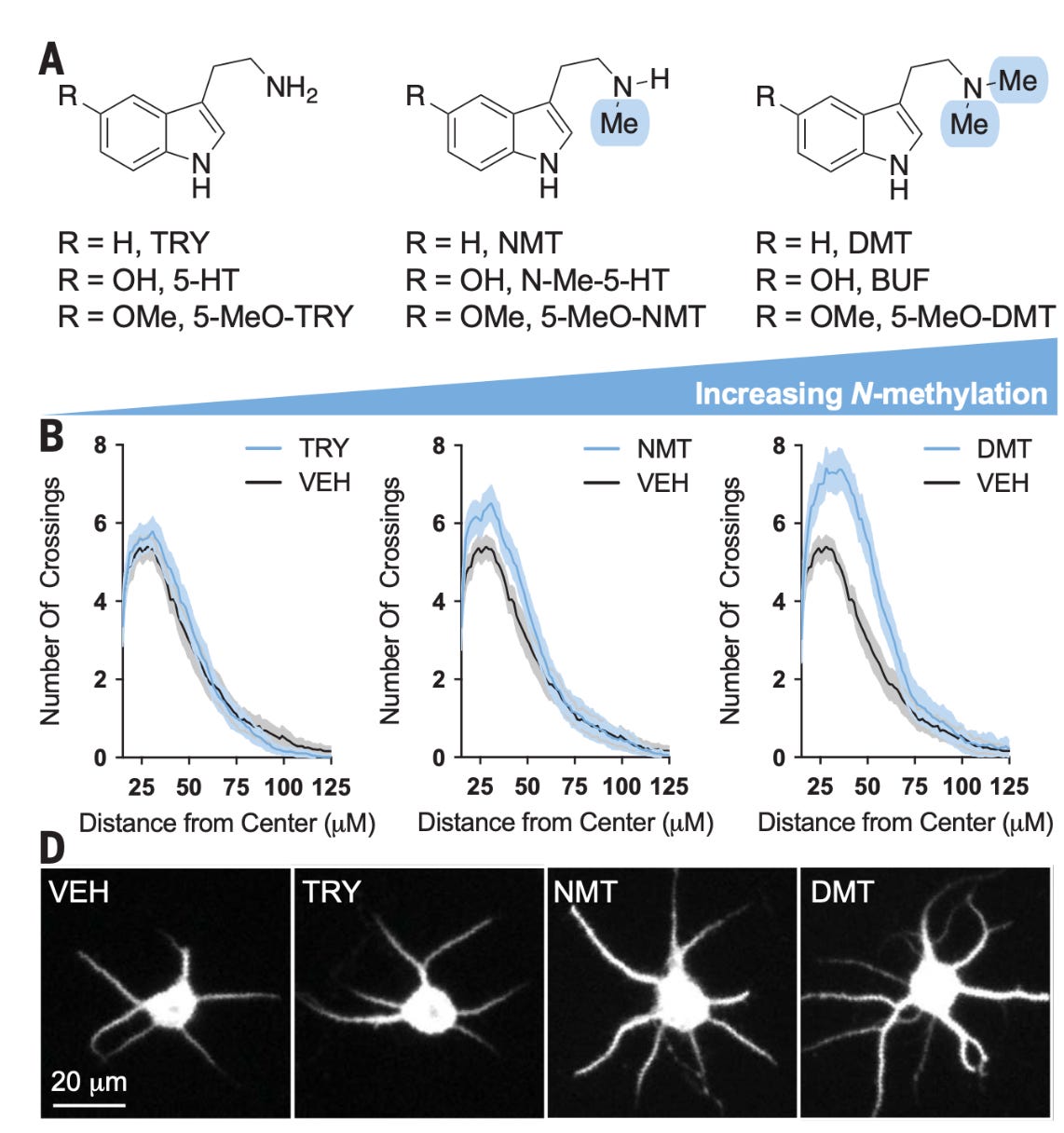
Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors [Vargas et al., Science, 2023]
Why it matters: Classical psychedelics such as DMT and LSD promote neuroplasticity, but the exact mechanism is poorly understood. A paper in Science this week shows that lipophilic psychedelics induce neuroplasticity by diffusing across the cell membrane and binding an intracellular serotonin receptor. This finding helps explain why psychedelics promote rapid plasticity and traditional SSRIs do not.The therapeutic mechanism of psychedelics in major depression is thought to be the induction of rapid and sustained neuroplasticity in the prefrontal cortex. Both psychedelics like ketamine and DMT and SSRIs act in part through activation of the serotonin receptor subtype 5-HT2AR, yet only psychedelics induce cortical plasticity. This week in Science, David Olson's lab showed that the paradox is due to intracellular vs extracellular 5-HT2AR. The group showed that increasing the N-methylation of 5-HT2AR agonists (which increases their lipophilicity and thus cell permeability) increased resulting dendritic arbor complexity in cultured neurons (dendrites are the input terminals to a neuron, and increases in their density and branching, important markers of neuroplasticity).
Further, across a range of compounds from serotonin (a polar compound that binds extracellularly) to DMT (non-polar compound that binds intracellularly), lipophilicity correlated positively with psychoplastogenecity (figure below). It turns out 5-HT2AR, a GPCR which is typically membrane-bound, is actually primarily intracellular. The authors did a number of other well-designed experiments to bolster their findings, including inducing membrane permeability using electroporation to show that non-membrane permeable compounds like serotonin can be induced to have a psychoplastogenic effect.
What we listened to
Notable Deals
Flagship's Pioneering Medicines to carve a new drug discovery path with Charles River's AI
Nanite Debuts AI-Driven Polymer Design Platform for Gene Delivery
In case you missed it
Medical AI Research Center (MedARC) has launched a novel, open, and collaborative approach to research dedicated to advancing the field of AI applied to healthcare!
What we liked on Twitter








Field Trip

Did we miss anything? Would you like to contribute to Decoding Bio by writing a guest post? Drop us a note here or chat with us on Twitter: @ameekapadia @ketanyerneni @morgancheatham @pablolubroth @patricksmalone