BioByte 028: the bacteria that may be behind rheumatoid arthritis, how drug discovery companies use GPT, all you need to know about protein engineering, first in class vs best in class, and more...
Welcome to Decoding Bio, a writing collective focused on the latest scientific advancements, news, and people building at the intersection of tech x bio. If you’d like to connect or collaborate, please shoot us a note here or chat with us on Twitter: @ameekapadia @ketanyerneni @morgancheatham @pablolubroth @patricksmalone. Happy decoding!
What a week! An RSV vaccine approval, much needed warnings on the AI race, a win for synthetic biology in cosmetics, and more. Let’s get into it.
What we read
Blogs
A Bacterial Culprit for Rheumatoid Arthritis [The Scientist, Drug Discovery News, Hannah Thomasy, 2023]
New microbe drop this week! Researchers recently identified a strain of Subdoligranulum that appears to be linked to the development of Rheumatoid Arthritis (RA). Using blood screening methods, researchers evaluated blood donated by people at risk of RA, with early-stage RA, or with RA-associated antibodies. The teams then evaluated whether any of the antibodies targeted human intestinal bacteria, after which, they seqeuneced bacterial species with attached antibodies, which revealed high reactivity with Lachnospiraceae or Ruminococcaceae species. Further stool analysis uncovered two types of Subdoligranulum bacteria as potential candidates for driving RA development.
The reason I think this line of research is particularly exciting is that it could very well get at an actual beginning of the disease.
- Rabi Upadhyay, NYU Grossman School of Medicine
Despite yet another compelling link between the microbiome and autoimmunity, this Subdoligranulum strain was only in 16.7% of people at risk or with early-stage RA, indicating that this strain is likely not the sole driver of disease. More work to be done!
Drug discovery companies are customizing ChatGPT: here’s how [Neil Savage, Nature Biotechnology, 2023]
We’ve heard about how our friends and colleagues are using ChatGPT and LLMs in various ways, but how are biotech companies using it?
Bioxcel Therapeutics, which uses AI for repurposing drugs that were shelved in phase 2 or 3 trials, or after approval, is considering LLMs to pick out winners from different databases. To ensure accurate results, and avoid hallucinations they use ‘evidence surfacing’, where a reference is provided after every assertion.
Insilico Medicine uses ChatGPT to interact with their target discovery platform to augment the relationships provided by their knowledge graphs. Instead of clicking away to discover new relationships between nodes, they just ask a question and compose text the scientists can understand.
Exscientia uses LLMs to translate ordinary English statements into carefully structured, mechanistic assertions that help generate their knowledge graphs
Open Review of “A somato-cognitive action network alternates with effector regions in motor cortex” [Muret et al., Diedrichsen Lab, 2023]
In last week’s edition of the newsletter we discussed a recent Nature paper that argues for a new model of functional and anatomical organization in the human primary motor cortex. This week, Jorn Diedrichsen’s lab published an open review of the paper calling for a more measured interpretation of the data. We are highlighting the open review to highlight a trend we are huge fans of: publishing reviews of scientific papers openly for all to read and discuss, and not hiding behind the cover of anonymity typical of the peer review process. We hope this is a trend that continues.
The first objection of the open review is that the classic model presented in the Nature paper (a linearly arranged motor homunculus, where each body part is controlled by a distinct sub-region of primary motor cortex) is overly simplified, and in fact there has never been much empirical evidence for this model. That the model has survived this many years is likely due to its simplicity, not its accuracy or comprehensiveness.
The main objection to the Nature paper is whether the primary claim of the existence of “inter-effector regions” within motor cortex that are responsible for mind-body integration and integrating goal-oriented action planning with the motor programs required for executing movement is supported by the data. For example, the authors of the review raise an important concern in the fMRI field called reverse inference, or the fallacy of inferring function or mental states from neuroimaging data without establishing causality, for example by using an interventional method like TMS to disrupt neural activity and observe the effect.
Single Cell Sequencing For Everyone [Derek Lowe, In the Pipeline, 2023]
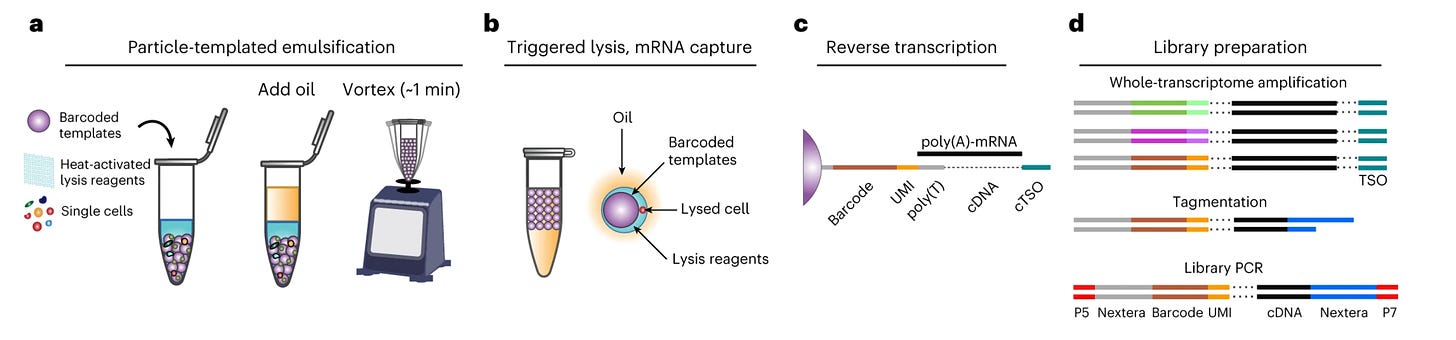
If you’ve done a single cell sequencing run, you know how expensive it can get. Single cell sequencing provides an incredible amount of data but comes at a hefty price given you have to physically look at each cell individually. Typically, single cell technologies have relied on some combination of microfluidics for cell sorting and DNA barcoding (labeling each cell with a unique DNA sequence). However, both are time-consuming and costly. A new paper from UCSF details a system that may potentially bring the costs of microfluidics separation down using “particle-templated instant partition sequencing” or PIP-seq. Think of the process as microfluidics in a tube, allowing the researcher to forgo having to use an expensive microfluidics device. The process is shockingly simple—add a layer of oil to a tube with barcoded beads and cells and vortex to emulsify. The result should be a single bead and single cell in each droplet, which can be lysed and processed as needed.
Academic Papers
First-in-class versus best-in-class: an update for new market dynamics [Spring et al., Nature Reviews Drug Discovery, 2023]
Why it matters: This update provides evidence on how assets perform depending on their mechanistic asset class, which can inform which drugs to prioritise to optimise value capture.In this fascinating update on the 2013 study by BCG, the authors discuss how market dynamics for drugs with the same mechanism have changed. How much is commercial success due to therapeutic advantage, launch timing and commercial acumen?
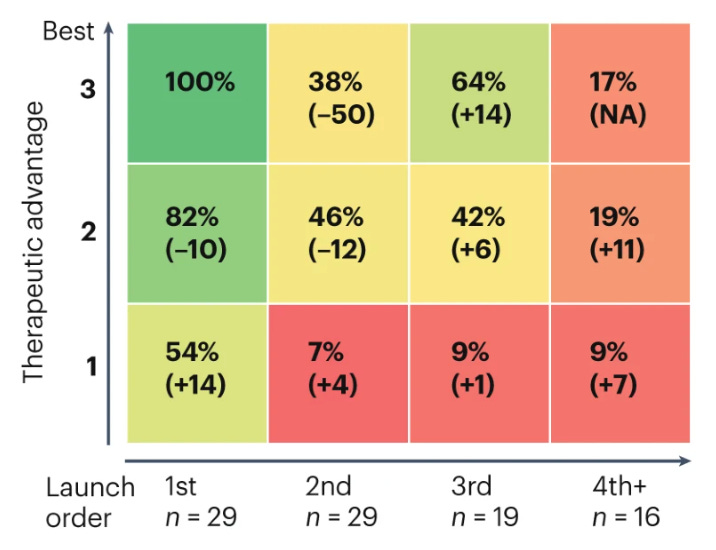
This figure shows the % of value captured when normalised to best-in-class and first-in-class segment in the top left. Here is a summary of the main findings:
products that are first-to-launch increasingly tend to perform better, and the advantage of being first over second has substantially increased
second-to-launch, but clearly best-in-class, products capture only 38% of the value that a first-to-launch and best-in-class product does.
first-to-launch products that received the middle score in therapeutic advantage garner 82% of the value compared to a first-and-best product, over twice as much as a second-and-best product
first-to-launch products with the lowest therapeutic advantage score capture 54% of the value of a first-and-best product, a 14% increase on the previous analysis.
this analysis also indicates that third and later entrants have captured more value than they did in the past
Therapeutic area has a significant impact in the value capture of later entrants:
oncology favors first-to-launch products. First-in-class oncology products have a significant advantage over followers, as long as they have at least the medium therapeutic advantage score.
outside of oncology, later entrants are more able to capture value especially in diseases where patients tend to cycle through multiple treatments, such as migraine, psoriasis and other inflammation-driven diseases
in classes covering multiple indications, first-to-launch therapeutically competitive or best-in-class products tend to be considerably more successful, partly through faster indication expansion
The authors recommend three strategies for later entrants:
launch with a best-in-class profile very shortly after or with a much better therapeutic profile than the first entrant
expand the addressable market through more valuable indications/sub-indications
use an alternative modality with an improved product profile
Engineering protein-based therapeutics through structural and chemical design [Ebrahimi and Samanta, Nature Comms, 2023]
Why it matters: Protein engineering as a field has dramatically accelerated in the last five years with machine learning applications and better understanding of structure/folding. This paper comprehensively reviews the latest advances in protein design as they relate to engineering therapeutics. A review out this week in Nature Communications surveys the most common approaches as well as recent cutting-edge methods for designing and engineering protein therapeutics. As the field has matured, there has been an increased appreciation for the importance of optimizing properties beyond just binding affinity for a therapeutic target, including stability (susceptibility of proteins to aggregation, denaturation, or degradation) and immunogenicity (figure below).
There are a couple exciting new areas to highlight. While proteins typically can only act extracellularly via a cell surface receptor, a number of emerging approaches have been developed to target proteins intracellularly, including cell-penetrating peptides, supercharging, and dense DNA grafting.
Supercharged proteins are highly charged proteins that have several desirable properties, including resistance to aggregation, and the ability to act intracellularly by binding to cell-surface proteoglycans. Supercharged proteins can be engineered using mutagenesis. Incorporation of unnatural amino acids into protein structures is another emerging modality, with advantages such as site specific PEGylation (tagging with polyethylene glycol to reduce clearance and degradation of a protein) for optimal improvement in protein PK, or attachment of fusion proteins.
What we listened to
Notable Deals
Orbital Therapeutics launches to advance RNA therapeutics and lands a strategic partnership with Beam Tx.
Revela gets acquired by Il Makiage parent company Oddity for $76M.
In case you missed it
Godfather of AI, Geoffrey Hinton has left Google due to concerns about the potential for generative technology to cause harm. Fascinating perspective from him in the New York Times.
GSK’s RSV vaccine gets the green light from FDA
What we liked on Twitter
Biotech monopolies and opportunity for competition @johncumbers
How AI can transform scientific publishing @emollick
Biases in CRISPR screens? Tweetorial on Recursion’s new paper @ImranSHaque
Video breakdown of ML x protein interactions paper @juliabauman2
The Merck acquisition of Prometheus Bio is a win for techbio @ElliotHershberg
TAME trial on metformin @JocelynnPearl
Events
SynBioBeta Happy Hour by Cantos, Bits in Bio, and Savills. [May 23, 2023. San Francisco, CA]. Reply to this email for invite.
BIO International Conference [June 5-8, 2023. Boston, MA]
Field Trip
The history behind your favorite video game is more complicated than you might think…
The days are long but the decades are short. Sam Altman on turning 30 (from 2015)
Did we miss anything? Would you like to contribute to Decoding Bio by writing a guest post? Drop us a note here or chat with us on Twitter: @ameekapadia @ketanyerneni @morgancheatham @pablolubroth @patricksmalone