BioByte 54: reprogramming therapies with mRNA, building bioweapons with chatbots, using active learning to traverse the chemical space, and more
Welcome to Decoding Bio, a writing collective focused on the latest scientific advancements, news, and people building at the intersection of tech x bio. If you’d like to connect or collaborate, please shoot us a note here or chat with us on Twitter: @ameekapadia @ketanyerneni @morgancheatham @pablolubroth @patricksmalone. Happy decoding!
Coming at you with a bite-sized BioByte just in time for some ~light~ weekend reading. As always, here are the highlights:
Reprogramming off-the-shelf cell therapies for cancer and autoimmune disease with mRNA
Cell stress with covalent inhibitors
AI building bioweapons
Chromatin reorganization in Treg cells
Voice user interface and speech models in the lab
Using active learning to expand training data
What we read
Blogs
mRNA’s next trick? Reprogramming off-the-shelf cell therapies for cancer and autoimmune diseases [Ryan Cross, Lei Lei Wu, Endpoints, 2023]
Carisma, Capstan, Oran and Moderna are pursuing a different avenue to CAR-therapies by transforming immune cells in vivo. Instead of collecting the patient's cells and engineering them out of the body, these companies are betting they can carry out a similar engineering process via an injectable mRNA therapy. One of the major setbacks for cell therapies has been the complexity in manufacturing operations, so in vivo CAR-based therapy could be the holy grail (with the exception that it requires multiple mRNA doses). Orna will be one of the first companies to test this methodology in humans in 2024 with its circular RNA technology which has longer expression versus linear RNA. Myeloid Therapeutics is already testing its therapy in humans.
Carisma and Moderna partnered to engineer macrophages in vivo, which are commonly found in the TME and therefore could be effective in the treatment of solid tumors.
As Dylan from Zetta Ventures mentions “…like all genetic medicines, delivery is going to be the major bottleneck and in vivo immunotherapies will require specificity to tissue and cell types that non-viral vectors like LNPs don't typically go to. Which means we're going to need some new delivery options…”. This will be a significant challenge for all of these companies. In their attempt to overcome this, Capstan incorporates antibodies in its LNPs to guide them to T-cells in the spleen.
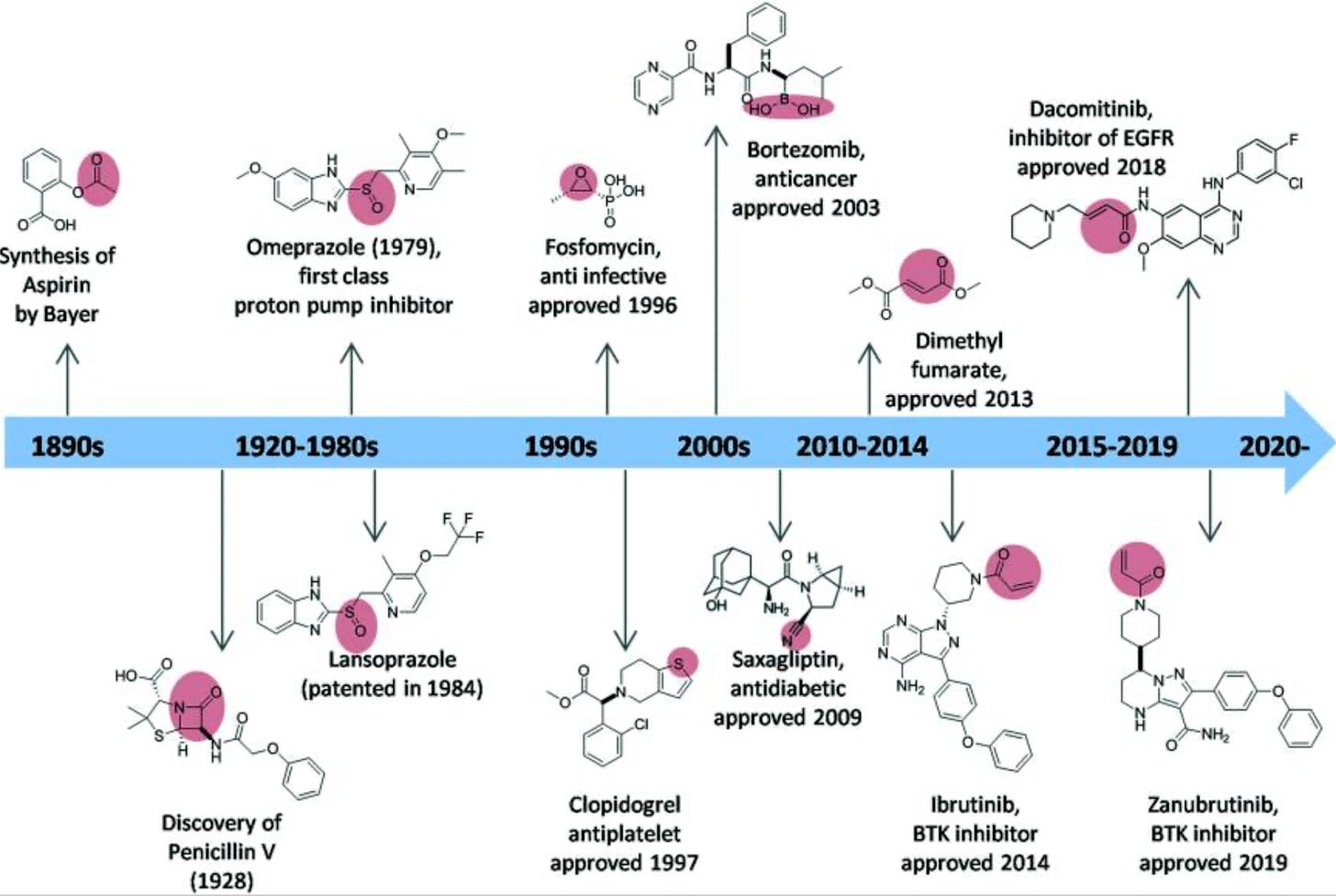
Covalent Complications [Derek Lowe, Nov 2023]
Covalent inhibitors are a class of drugs that form covalent bonds with their target molecules. Unlike non-covalent inhibitors, which bind reversibly to their targets through non-covalent interactions like hydrogen bonds or van der Waals forces, covalent inhibitors create a stable, irreversible bond with their targets. Some of the potential advantages of covalent inhibitors include increased potency, selectivity, and duration of action.
In a new blog this week, Derek Lowe discusses some recent concerning findings (e.g. this preprint from Keriann Backus’ lab at UCLA) about covalent inhibitors that suggest that the drugs trigger a range of cell stress responses. At issue is the lack of selectivity of cysteine-targeting covalent compounds (the most common type of covalent inhibitors used). Off-target effects that have been observed include global changes in cellular proteostasis, including increased ubiquitylation, proteasome activation, depletion of host proteins (notably in the nuclear pore complex), stress granule formation, and protein aggregation into aggresomes. Derek argues that the extent of cellular stress responses and mechanistic implications of these compounds have been underappreciated, and the findings call for further research from the covalent inhibitor field.
Historical overview of covalent inhibitor drugs and their approval dates. From Sutanto et al., RSC Med Chem, 2020.
Can Chatbots Help You Build a Bioweapon? [Batalis, November 2023]
The article by Steph Batalis raises concerns about the potential of AI, and particularly chatbots, to lower the barrier for constructing biological weapons by providing step-by-step instructions for engineering pathogens. While acknowledging the seriousness of this threat, Batalis also points out that scientific knowledge is already widely available without the aid of chatbots. The piece argues that the perception of chatbots as gatekeepers of specialized information may be overstated, considering that scientific information is freely accessible for various educational purposes and that biosecurity measures need to account for this reality.
Batalis suggests that in addition to implementing safeguards in chatbot development, a comprehensive biosecurity strategy should include measures like screening DNA orders and balancing security with scientific openness, especially for dual-use research. The article mentions the U.S. President's executive order on AI, which includes a DNA screening requirement, as a positive step, but calls for broader application. The author emphasizes the importance of fostering the next generation of biological inventors and not stifling innovation and economic growth in the bioeconomy, suggesting that future policy should balance the dissemination of science with the necessary guardrails against its misuse.
Academic papers
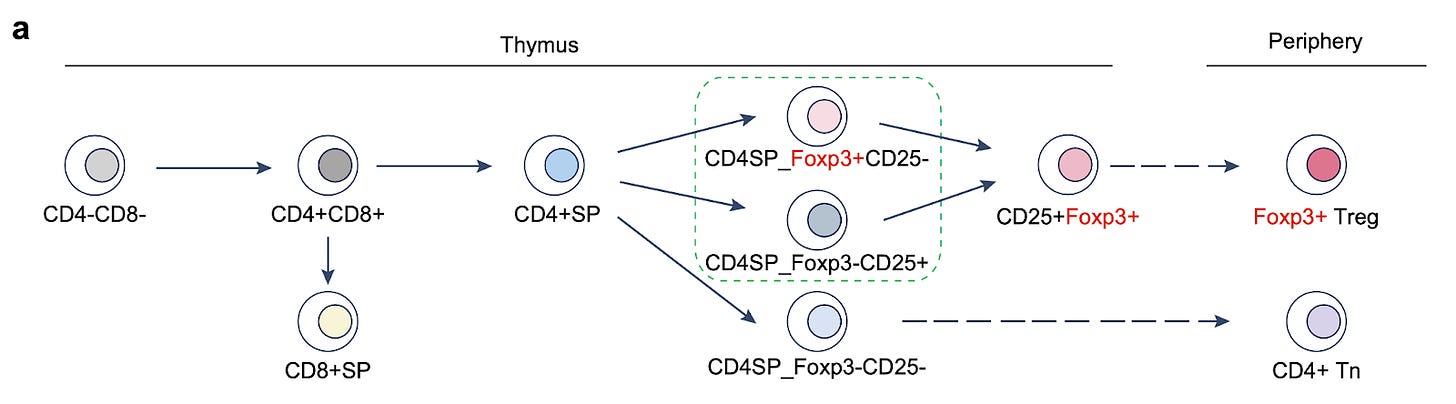
Foxp3 orchestrates reorganization of chromatin architecture to establish regulatory T cell identity [Liu et al., Nature Communications, Nov 2023]
Why it matters: The latest study from Jesse Dixon’s lab at the Salk finds that the transcription factor Foxp3 plays a crucial role in the development of regulatory T cells (Tregs) by reorganizing 3D chromatin structure. This reorganization is vital for the unique gene expression profile and function of Tregs, which are essential in maintaining immune tolerance and preventing autoimmune diseases.Tregs are a subpopulation of T cells generated in the thymus (see the T cell lineage map in the figure below), specializing in suppressing excessive immune responses. Chromatin conformation reorganization plays a crucial role in gene expression and cell lineage specification across cell types including Tregs. To map 3D chromatin organization during Treg cell differentiation, Dixon’s group used a technique called Hi-C (high-throughput chromosome conformation capture). Foxp3 was identified as critical for establishing Treg-specific 3D chromatin structure. In the absence of Foxp3, Treg-specific DNA contacts are significantly reduced, and Foxp3 knockout mice showed increased immune system activation. The study highlights the underappreciated role of Foxp3 in modulating Treg-specific 3D chromatin structure formation. These findings have important implications for the development of drugs that target Tregs, for example by designing epigenetic therapies that specifically promote (to treat autoimmune disease) or inhibit (to treat cancer) chromatin reorganization via Foxp3-mediated mechanisms.
An expandable voice user interface as lab assistant based on an improved version of Google’s speech recognition [Avila Vazquez et al, Nature Reports, November 2023]
Why it matters: Rainbow, a customizable voice user interface for scientific labs, demonstrates that existing speech recognition models can be leveraged for lab settings without costly retraining. The customizable nature of the platform allows optimizing for specialized vocabulary and tasks in any research domain. By automating routine bench activities through voice, Rainbow has the potential to improve laboratory workflows. The study provides a generalizable methodology for constructing customizable assistants from accessible components.This work presents Rainbow, a customizable voice user interface for scientific labs built using free software components. Rainbow utilizes Google Translate for speech processing and the AutoIt scripting language to control actions. It performs Windows automation tasks, lab-specific skills like protocols and calculations, and can integrate instruments.
Testing shows Rainbow recognizes commands with 91.3% accuracy, significantly higher than Google Translate alone at 85.1%. Users can expand Rainbow by adding customized vocabularies and links via a graphical interface, further improving individual accuracy to 98.6%. Being open source and local, Rainbow provides data privacy advantages over commercial assistants.
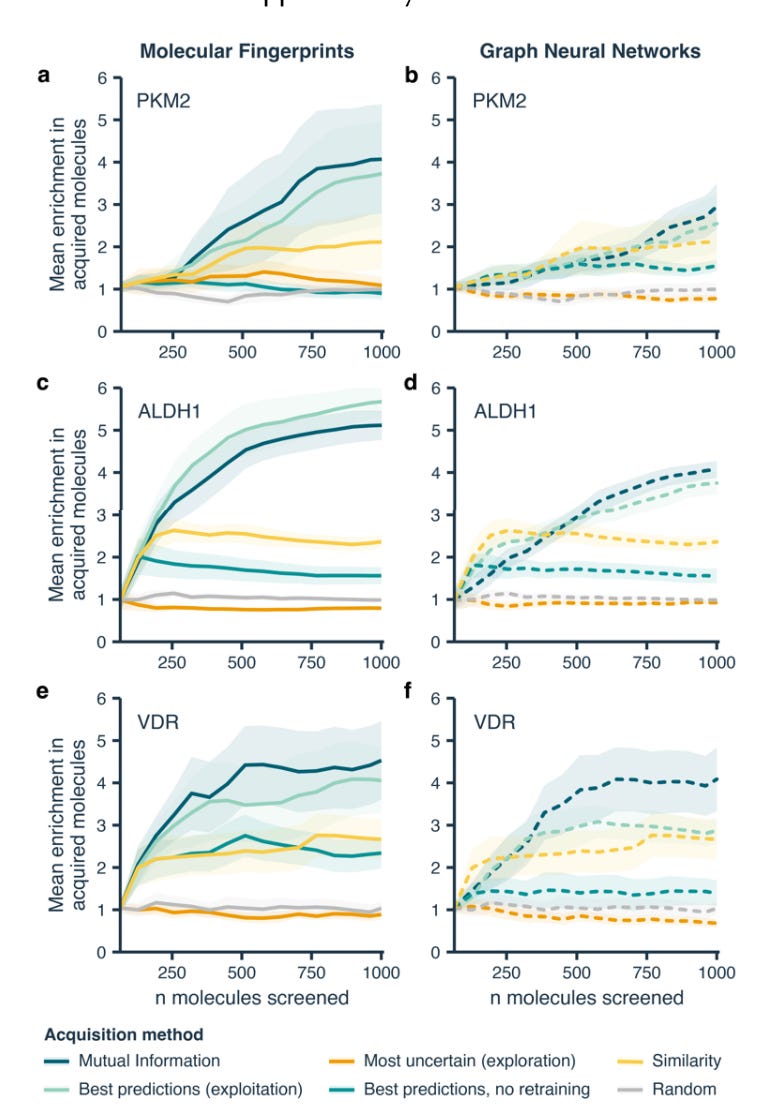
Traversing Chemical Space with Active Deep Learning [van Tilborg et al., ChemRxiv, Nov 2023]
Why it matters: Machine learning methods for drug screening are becoming increasingly prevalent but are limited by training data—namely low molecular diversity and small dataset sizes. This paper suggests using active learning to iteratively update a given model by screening a limited set of molecules at a time to grow the training data set instead of falling back on one-shot screening. The authors compared six different active learning strategies and found that the most important factor influencing hit discovery is the way in which you acquire molecules for each cycle. The workflow involved iteratively querying a screening library of 100,000 molecules, starting with sets of 64. The screening process involved training on all available data, acquisition of the 64 molecule sets, testing on bioactivity predictions, and updating where test molecules were added to the training set. The six strategies studied for the acquisition stage are as follows:
Molecular similarity—selecting those with highest structural similarity
Exploitation—selecting best model performance
Exploration—selecting most uncertain predictions (to patch knowledge gaps)
Mutual Information—selecting lowest mutual information
Exploitation without retraining—selecting on just the start data-set (one-shot)
Random acquisition—selecting from screening library at random for control
Only two deep learning models were tested—the classic neural network and GNN. The iterative approach found helps adjust the models in steps rather than all at once at the end of the screening process. Overall, the findings in this study advocate for active deep-learning augmented strategies in drug discovery when there is low levels of training data to begin with. The results also suggest it doesn’t matter whether models start with low-diversity in the training data—this can be accounted for after a few cycles. Very interesting how models can discover new chemistry over time.
Notable Deals
BioNTech: COVID’s out, oncology is in
BNTX recently held an innovation day, showcasing one of the most expansive, diverse and innovative oncology pipelines in biotech. Typically biotechs stick to a specific type of modality and master it’s discovery and development. BNTX however has multiple assets across key platforms (IO, ADCs, CARVac, FixVac, iNeST, NEO-STIM and RiboMabs) both in-licensed (ADCs from Duality Bio and MediLink and IO from (OncoC4 nd Biotheus) as well as their own internal efforts. They have also invested heavily in AI (via Instadeep). This breadth allows BNTX to personalise therapies for patients, offer novel combinations that focus on novel targets and diversify risk. To realise these aims, careful execution will be key.
Pharma hype cycles:
Obesity: AstraZeneca is paying china-based Eccogene $185M upfront for the rights to its oral once-weekly GLP-1 drug, a compelling alternative to current injectable therapies. With GLP-1 drugs touted to have peak annual global sales of $90M and AstraZeneca’s heavy cardiometabolic footprint (Brilina, Farxiga), it makes sense that they rejoin the race.
ADCs: BMS is paying Orum Therapeutics $100M upfront for its novel ADC asset that attaches a protein degrader to an antibody rather than deploying chemotherapy.
Nvidia invests in Terray Therapeutics
Terray Tx: Biotech with an end-end platform that integrates automated chemical experiment data and generative AI for the design of novel small molecule drugs.
Investment: NVentures has invested an undisclosed amount into the AI-biotech.
Collaboration: Terray will leverage Nvidia DGX Cloud, its software stack and compute expertise to develop a chemistry foundation model for small molecules. A part will be available on Nvidia’s BioNeMo (a cloud service for AI drug discovery applications).
Rationale: Nvidia has made a series of investments into companies such as Recursion and Charm Therapeutics, on its quest to catalyse the AI future of drug discovery and ensure it happens with Nvidia’s stack and ecosystem.
Roivant CEO Matt Gline’s favorite deal
“Our Sumitomo deal was special for me. It was truly mine – I went to Japan 15 times in like 10 months, and they came here as often. We needed each other. I built some real, durable, lasting relationships that spanned many continents and time zones. It was an incredibly important deal for Roivant, and it was clarifying doing work that really, really mattered. And it was just fun to pull together. We had a great team working on it.”
What we listened to
Jason Carman deep dives into biotech, starting with Octant.
In case you missed it
What we liked on Twitter
Full thread on the book release
Field Trip
Update 11/10/2023: added Myeloid Therapeutics as one of the first to test in vivo CAR in humans.
Did we miss anything? Would you like to contribute to Decoding Bio by writing a guest post? Drop us a note here or chat with us on Twitter: @ameekapadia @ketanyerneni @morgancheatham @pablolubroth @patricksmalone