BioByte 004
detecting Alzheimer's from retinal scans, programmable RNA sensors, making research open-source, and digitizing biotech
Welcome to Decoding TechBio, a writing collective focused on the latest scientific advancements, news, and people building at the intersection of tech x bio. Subscribe for free to read our weekly review and deep dives on areas we’re excited about. For more on us, check out our mission statement here. If you’d like to connect, please shoot us a note at decodingtechbio@gmail.com. Happy decoding!
Cuffing season is coming up and biology and engineering are definitely dating right now. This past week was another whirlwind for bio, and again, we’ve got you covered.
But first! A few announcements. First, we’ve finally decided what our first long-form articles will cover:
Open Source x Biology
Biosecurity
Transformers in Biology
We’re hoping to hear from our readership and the greater TechBio community as we author these pieces. Drop us a line at decodingtechbio@gmail.com if you’re interested in contributing.
Secondly, a few of us are organizing a happy hour for founders, operators, scientists, and investors in NYC on November 9. If you happen to be in the area, RSVP here and come through! We’d love to meet you.
What we read
Academic Papers
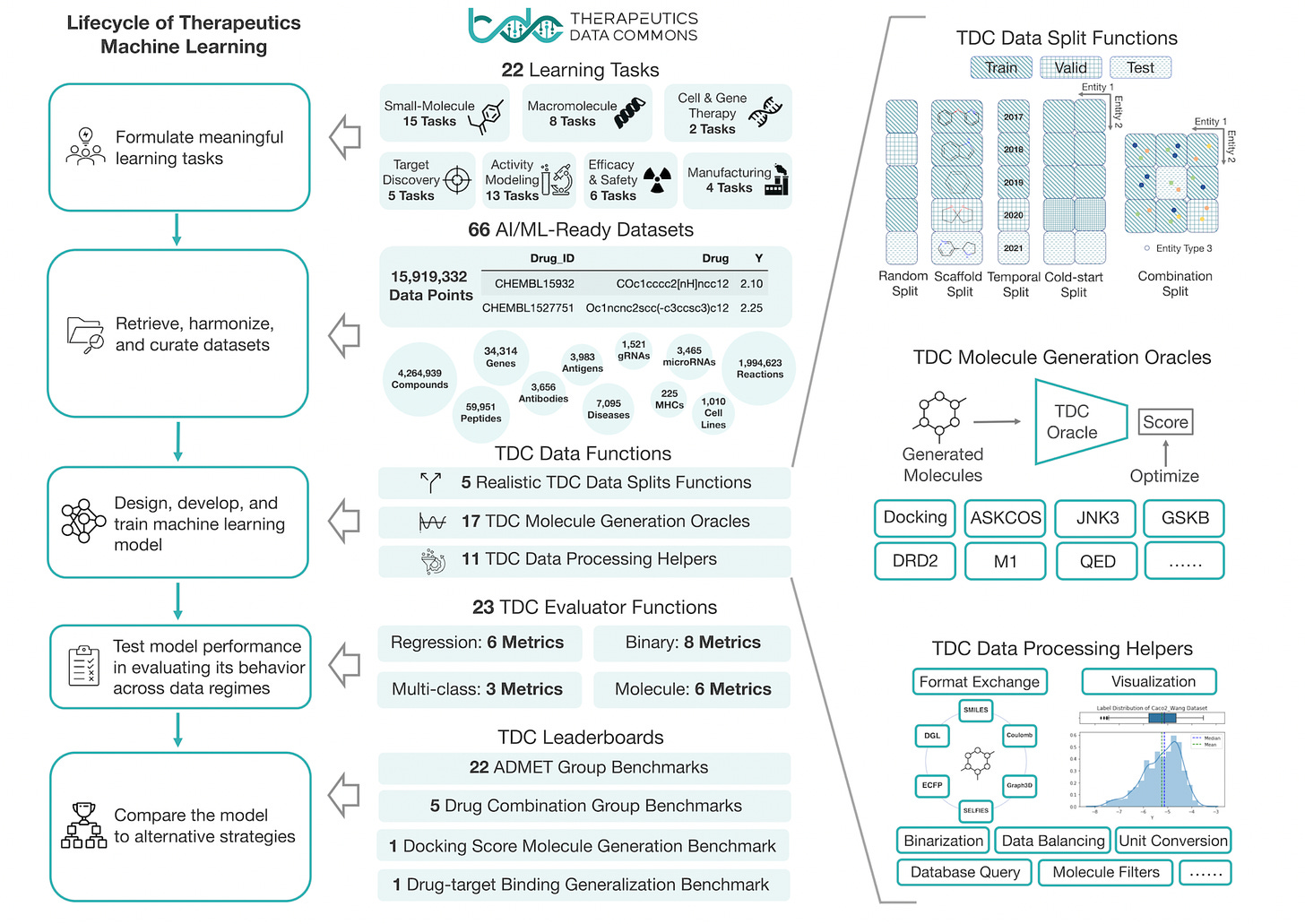
Artificial intelligence foundation for therapeutic science [Huang et al., Nature Chemical Biology 2022]
The Therapeutic Data Commons is an initiative for the democratization and evaluation of AI capabilities in drug development. Resources include curated benchmarks, tasks, and datasets, and a suite of tools, libraries, leaderboards, and community resources. This recent paper in Nature Chemical Biology provides an overview of the TDC, which contains 66 AI-ready datasets across 22 tasks in drug discovery across a range of therapeutic modalities. TDC also contains a python library for data pre-processing and model evaluation for method development. These resources will provide a sandbox for rigorous testing of ML methodology, and should help improve reproducibility and collaboration in the field. Happy hacking!
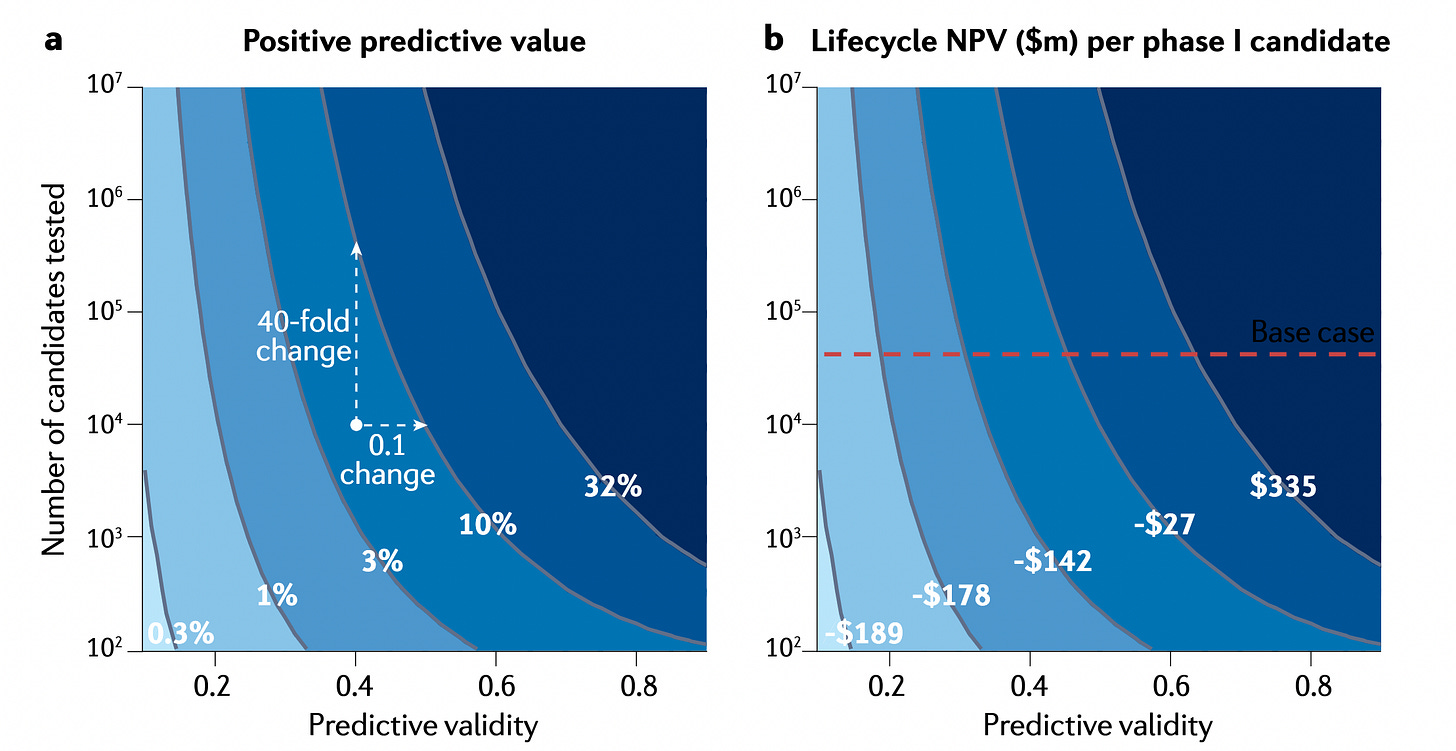
Predictive validity in drug discovery: what it is, why it matters and how to improve it [Scannell et al., Nature Reviews Drug Discovery 2022]
Latest from Jack Scannell on the importance of predictive validity of disease models in drug development. Seems obvious, but even so this fact is likely under under-appreciated. Using decision theory, the authors adopt the perspective that disease models and screens and pre-clinical assays are used as decision tools to decide whether to advance or shelve a therapeutic candidate. The quality of decision-making in drug development is highly sensitive to the ability of these tools to predict clinical utility of a potential drug. For example, for a given change in predictive validity of an assay, there is a greater improvement in R&D productivity relative to increasing the number of candidates tested (see figure below). In other words, all else being equal, a clinically/biologically relevant assay is much more valuable than increasing the number of shots on goal by increasing the number of drug candidates tested. The paper is packed with many more quantitative and anecdotal insights, and is well worth the read.
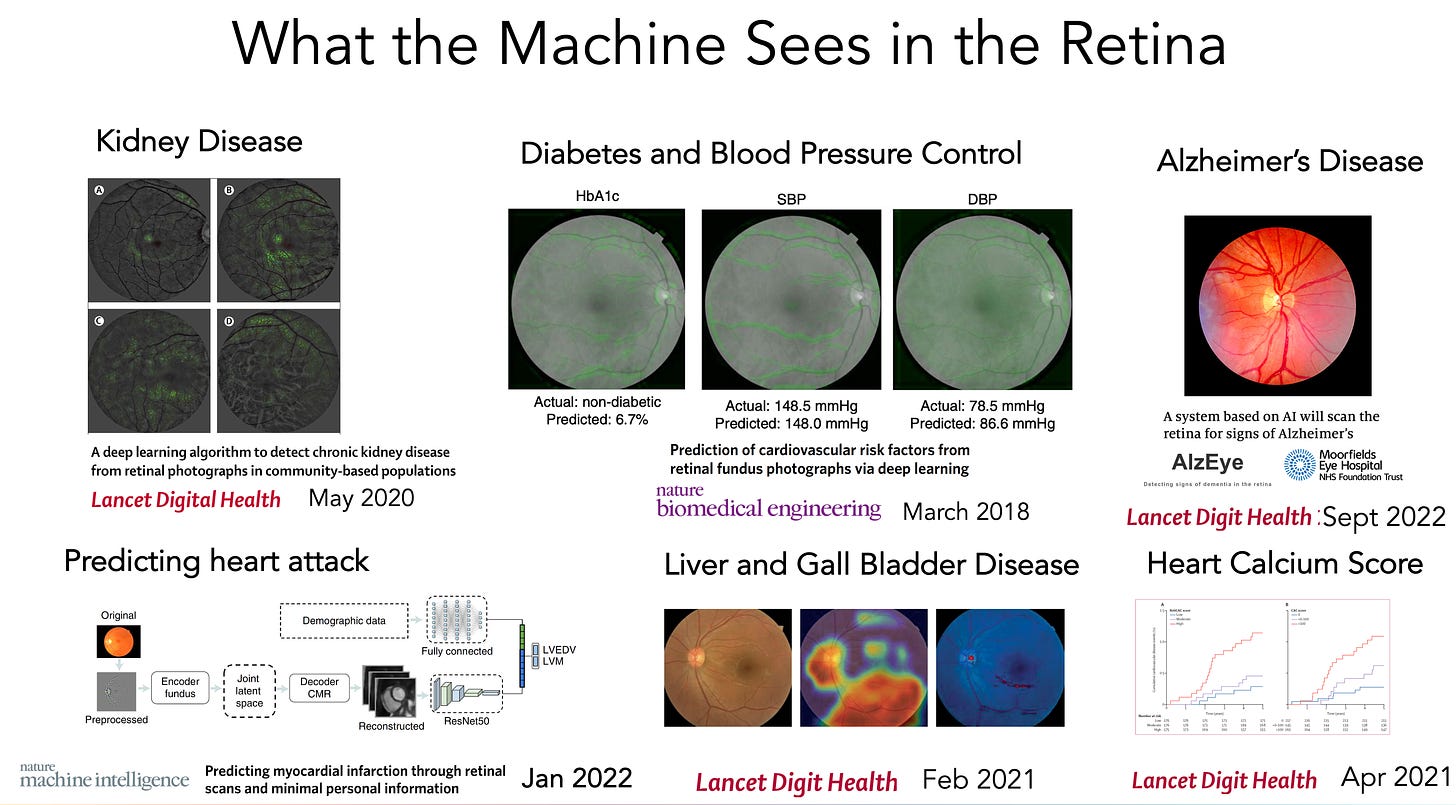
A deep learning model for detection of Alzheimer's disease based on retinal photographs: a retrospective, multicentre case-control study [Cheung et al., The Lancet Digital Health 2022]
Retinal biomarkers are an active area of research for neurodegenerative diseases, and notably, Alzheimer’s, based on a growing body of evidence suggesting that Alzheimer’s disease pathophysiology is associated with retinal changes including amyloid β deposition, neurodegeneration, vascular pathology, and inflammation. Given the large availability of retinal data assets and the feature-rich nature of the retina, applying hypothesis-free computer vision approaches shows promise for retinal biomarkers, which, if developed effectively, could be extremely useful clinically as a cost-effective, non-invasive diagnostic and prognostic tool. In this recent paper by Cheung et al., researchers demonstrate compelling accuracy and AUC leveraging images alone, which poses questions about whether combinatorial approaches (e.g., retinal images plus clinical or molecular data) could unlock entirely new, high-fidelity diagnostics for Alzheimer’s.
For additional reading on other retinal biomarkers, check out this paper on AI-enabled retinal analysis for the prediction of myocardial infarction (heart attack) and stroke. And, here’s a great overview of machine learning applied to retinal images across indications from Eric Topol’s recent blog post, “The Amazing Power of Machine Eyes”:
Polygenic enrichment distinguishes disease associations of individual cells in single-cell RNA-seq data [Zhang et al., Nature Genetics 2022]
Single-cell sequencing methodologies are all the rage in biology right now. First discovered in 2009, single-cell RNA sequencing (scRNA-seq) technology strives to understand the complexity of RNA transcripts within individual cells, as well as at the population level spanning tissues, organs, and organisms, with significant applications in understanding the molecular underpinnings of disease.
In parallel, polygenic risk scores leverage vast amounts of genomic data to understand which genomic variants tend to be found more frequently in groups of people with a given disease (note: there can be hundreds or even thousands of variants per disease).
This recent paper by Zhang et al. introduces the single-cell disease relevance score (ScDRS), an approach that combines scRNA-seq with polygenic disease risk scores at single-cell resolution. This methodology provides valuable insight into how risk variants identified via genome-wide association studies (GWAS) impact tissues and cell types. The team applied scDRS to 74 diseases/traits in conjunction with 16 scRNA-seq data sets and detected extensive heterogeneity in disease associations of individual cells within classical cell types.
Modular, programmable RNA sensing using ADAR editing in living cells [K. Eerik Kaseniit et al., Nature Biotech 2022]
As mentioned above, single-cell transcriptomics is the state-of-the-art method for cell type and state characterization (i.e. how do cells differ in tissues and how cells ‘are’ in healthy or diseased states). Despite the importance in contemporary molecular biology, targeting cells on the basis of their RNA profile has remained challenging. The authors succinctly describe why this would be hugely impactful:
RNA sense–response systems would enable the identification and destruction of harmful cells (for example, in the contexts of cancer and autoimmune disorders), or the experimental manipulation of specific cells in a complex ensemble (for example, the nervous and the immune systems)
Adenosine deaminases acting on RNA (ADARs) edit RNA, in an analogous manner to how CRISPR/Cas9 edits DNA. Building upon these enzymes, Eerik Kaseniit and company have engineered RADAR sensors: RNA sensors using ADAR.
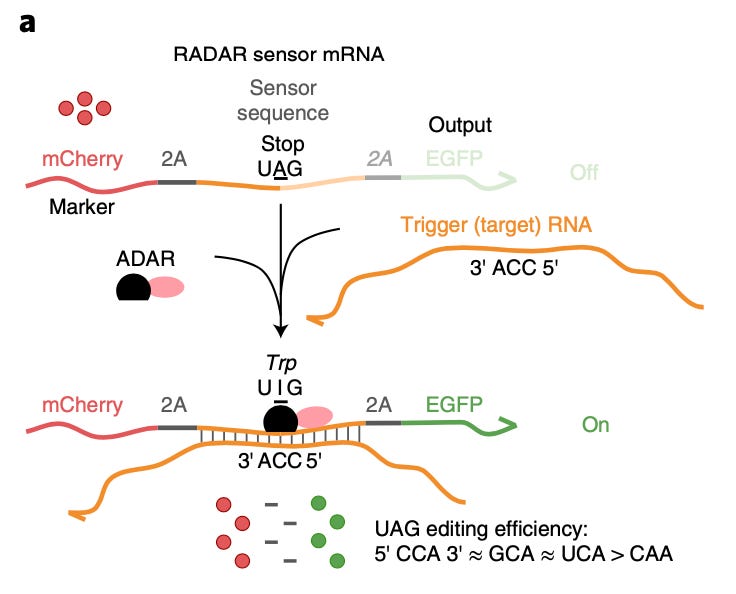
These molecular sensors utilize a ‘sensor sequence’ which binds to the ADAR enzyme and are complimentary to a ‘trigger/target sequence’. Should the latter sequence be present in the cellular environment, it triggers an edit and thus a detectable and measurable response. In the paper, the team demonstrates that RADARs are modular and programmable, and have multiplexing and logic capabilities.
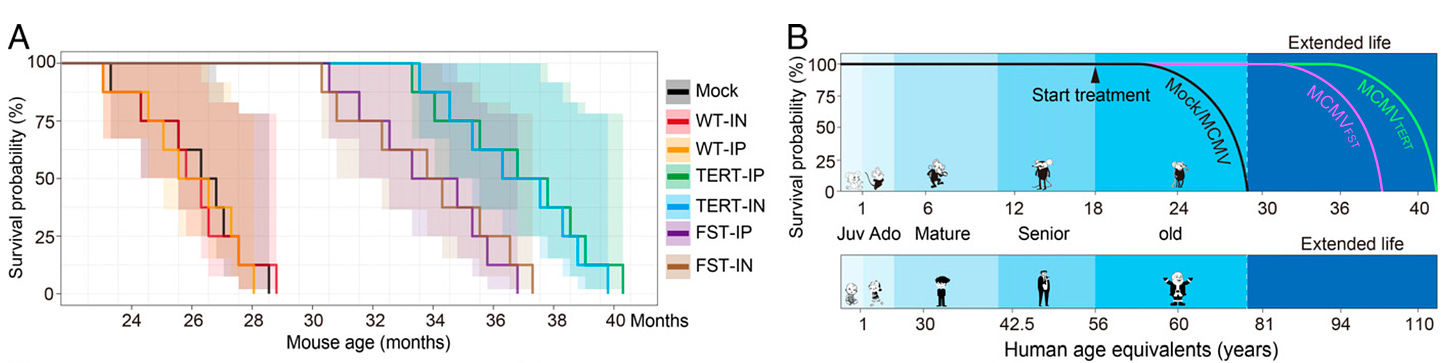
New intranasal and injectable gene therapy for healthy life extension [Dabbu Kumar Jaijyan et al., PNAS 2022]
The teams at Rutgers NJ Medical School, BioViva and Harvard showed that the high-capacity cytomegalovirus vector (CMV) can be an effective and safe gene delivery method for two proteins that can suppress or reverse the effects of aging in animal models. Congruent with other studies, proteins telomerase reverse transcriptase (TERT) and follistatin (FST) extended median lifespan. The intranasal delivery of the therapy performed equally in terms of safety and efficacy as its injectable counterpart. CMV’s advantage over other delivery vectors is that it:
has an ample cargo capacity and
enables efficient intranasal administration. Olfactory infection is associated with lower immunogenic effects than intraperitoneal inoculation.
Blogs
Nobel Prize awarded to three scientists for work in click chemistry, which links molecules quickly [Washington Post, October 2022]
Nobel Prize Alert! This past week, the 2022 Nobel Prize in chemistry was awarded to three scientists for work in a field known as click chemistry, which allows molecular building blocks to be snapped together like Lego pieces to create complex molecules with huge implications for pharmaceutical development, medicine and material sciences. Awardees included:
K. Barry Sharpless of Scripps Research
Carolyn R. Bertozzi of Stanford University
Morten Meldal at the University of Copenhagen
Click chemistry is a class of reactions commonly used in bioconjugation to join small molecules to biomolecules. Part of the power of click chemistry is that it is bioorthogonal - a type of chemical reaction that can occur in a living system without interfering with native biochemical processes. Thus, click chemistry can be used to monitor ongoing biological activity in living sysetms. The first click chemistry reaction was a copper-catalyzed azide-alkyne cycloaddition discovered by Meldal and Sharpless. Scientists quickly realized that by tagging molecules of interest with azide and alkyne and sprinkling a little copper on top, they could link molecules together to make larger structures. Bertozzi used click chemistry to track signaling molecules on the cell surface known as glycans. By linking glycans to an azide and adding an alkyne with a fluorescent tag, Bertozzi was able to track glycan movement under a microscope. Bertozzi’s lab used the technique to help elucidate the role of glycan signaling in immune response to cancer, and served as the scientific foundation for the biotech Palleon Pharmaceuticals.
Lilly inks $425M biobuck drug discovery pact with Schrödinger [Nick Paul Taylor, Fierce Biotech, 2022]
Using the machine-learning-enabled platform, Schrödinger can test billions of molecules in computational assays and, it claims, half the time taken to deliver a drug development candidate while improving on the properties achieved using traditional design processes. Adding to its growing number of partnerships, which already include Sanofi and BMS, Lilly has committed $425M in milestones to access small-molecule candidates against an undisclosed target. Given the it is a platform, not an asset development partnership, the upfront fee is thought to be small. The company, which generates revenues from its software and drug discovery work, has fared better than many pure-play biotechs in the bear market of 2022.
Time for Change [Dr. Jocelynn Pearl, Motif, 2022]
In this piece, Dr. Pearl previews the “new wave of activist scientists getting creative in the battle against traditional publishing.” In an industry governed by publish or perish dynamics and an admired (but feared) peer review process that benefits publishers more than scientists, emerging projects are exploring new and exciting models for scientific publishing (like this cool open-sourced peer review, PeeryView, and PubPub, an open-source, community-led end-to-end publishing platform for knowledge communities).
In addition to exploring new platforms for canonical scientific publishing processes, Dr. Pearl calls into question the very format of scientific articles - might there be ways in the 21st century to explain science? Or are PDFs really our best foot forward? What would it look like to leverage a smart manuscript (perhaps inspired by Balaji Srinivasan’s hierarchical organization of his latest book, The Network State)? We at Decoding TechBio view the PDF manuscript as yet another casualty in the skeuomorphic design reality — simply put, an underappreciation of the digital-first medium we now find ourselves in.
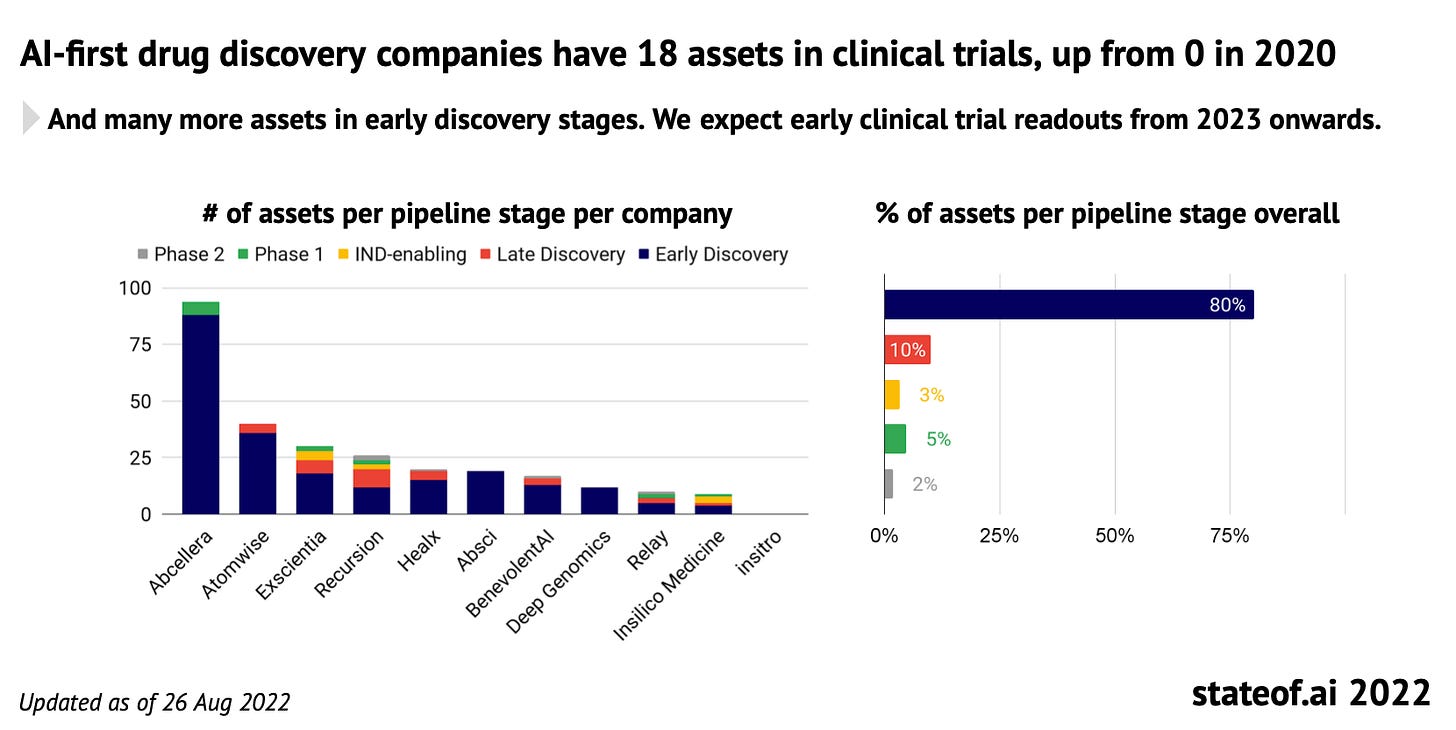
State of AI Report 2022 [Nathan Benaich and Ian Hogarth, Air Street Capital, 2022]
Air Street just dropped its newest edition of State of AI, covering the most interesting developments in AI in the past year. Here are some of the TechBio highlights, but make sure to visit the full report here.
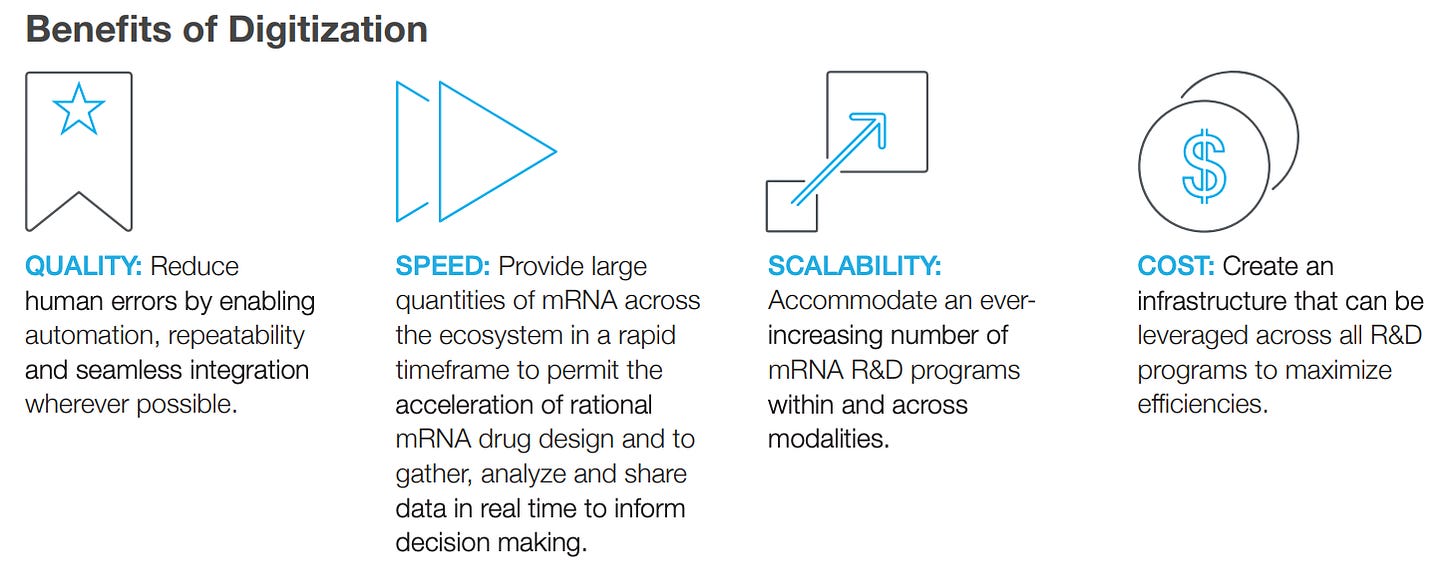
How Building a Digital Biotech is Mission-Critical to Moderna [Moderna, 2017]
We went back to our roots this weekend and re-read Moderna’s original whitepaper. Originally authored in 2017, it’s remarkable how foundational this piece has become. The piece highlights both the ways digital infrastructure supports key aspects of the research and development process — from the rational design of mRNA medicines and the acceleration of programs through research — as well as non-scientific functions like Human Resources, Finance, Infrastructure, and End User Computing.
Since Moderna’s inception, we have invested over $100 million on our digital technologies, robotics/automation, analytics, data science and AI. Given our growth, we expect we will invest more than $100 million in digital over the next 5 years.
White House’s Open Access Research Directive Scrambles Long-Entrenched Models, Raising Key Questions [STAT News, 2022]
The White House’s Office of Science and Technology Policy put out a memo with guidance on making federally funded research available without restrictions by the end of 2025. Before you get all excited, this doesn’t mean all research articles will suddenly be accessible to the general public with no problem. It seems like you’ll still have to follow new research back to the databases of the agency that funded the work before getting access. It still remains to be known how this new directive will affect journals, but it is a step in the right direction for open-source access to cutting edge science.
The future of open science, the seamless integration of data and information flow is a transformative future that we’re trying to get to at the end of the day,” said Lyric Jorgenson, the acting associate director for science policy at the National Institutes of Health. “We want to make sure all these steps that we’re taking aren’t fulfilling a requirement as much as they are really taking actual steps towards this new future that we’re trying to envision together.
Why Applying Machine Learning to Biology is Hard – But Worth It [Jeremy Lin and Nicole Neuman, Future]
In this piece, Jeremy Lin, Chief Scientific Officer at Freenome, a blood-based cancer diagnostics company, offers a real view into the benefits, challenges, and considerations of melding machine learning and multi-omics data and the multi-lingual teams required to bring these approaches to life. We share Jeremy’s excitement for this unique moment in biological sciences:
Outside of cancer diagnostics, what’s also really cool is the transition to building with biology instead of just reading and writing. I’m excited about the areas of synthetic biology where we’re using biology as a technology, whether it’s CRISPR or synthetic peptides or synthetic nucleotides. Leveraging biology as a tool creates expansive possibilities to completely transform traditional resource generating industries, from agriculture to energy. This is truly an amazing time to be a biologist! -Jeremy Lin, CSO of Freenome
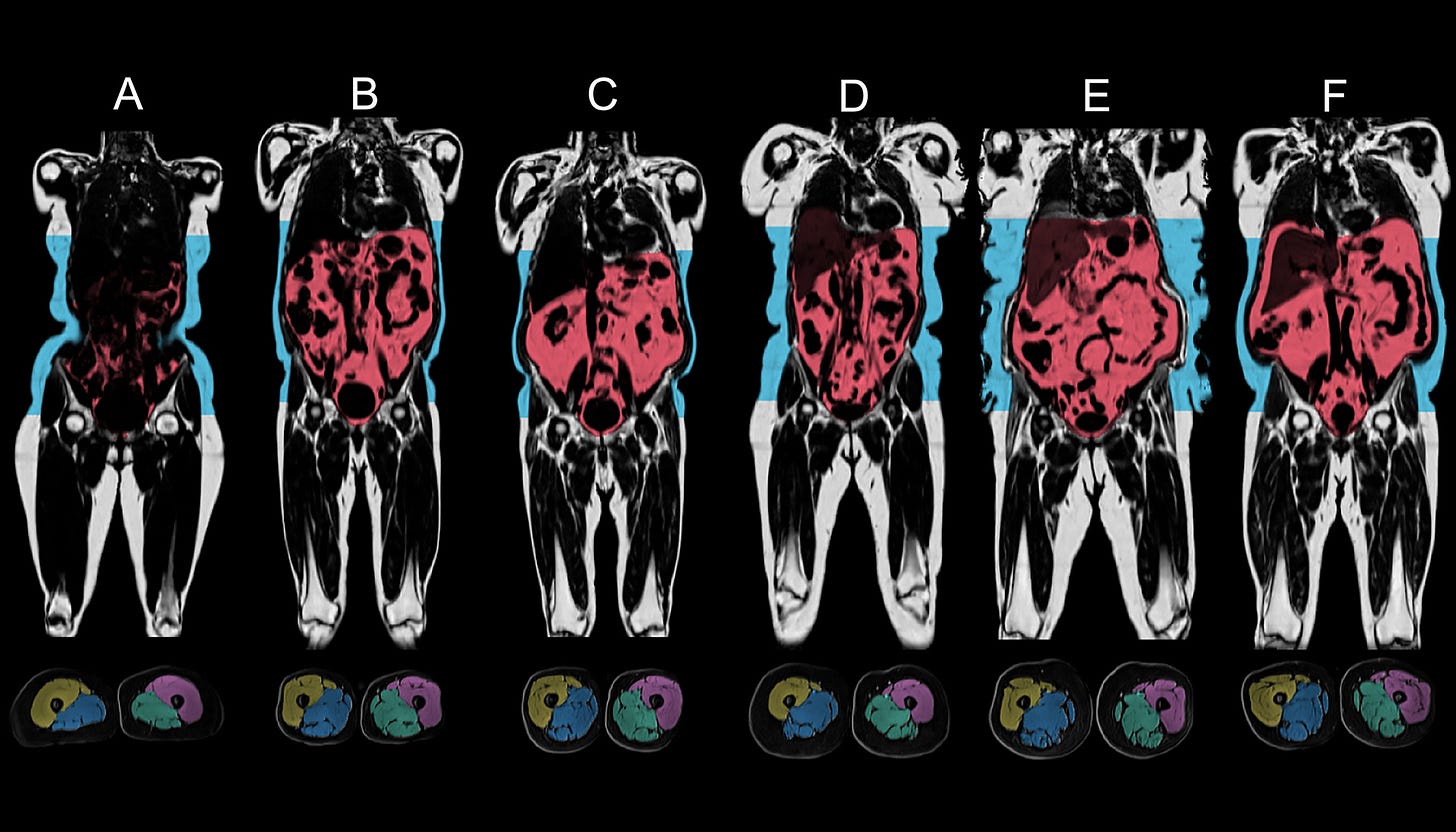
CZI Supports UK Biobank to Create World’s Largest Imaging Dataset To Understand Disease [Chan Zuckerberg Initiative]
Recently, the Chan Zuckerberg Insitute announced a collaboration with the UK Biobank to create the world’s largest imaging dataset to understand disease. The UK Biobank’s imaging work began in 2014 and already includes MRI data from the brain, heart, and abdomen, together with bone density and ultrasound scans of the carotid arteries, from over 50,000 participants, expected to eclipse 100,000 over the next two years.
This new £30m project will fuel a second phase that will facilitate repeat imaging on 60,000 participants two to seven years after their initial scan, resulting in a repeated set of highly-detailed, multi-organ images from a large cohort of participants, allowing researchers to assess changes in physiology over time. Combined with robust clinico-genomic data already maintained by the UK Biobank, when finished, this asset could offer a unique and multi-faceted view into disease pathophysiology and progression for a number of indications.
What we listened to
Great overview of the field of click chemistry and its applications following last week’s Nobel prize:
KdT Ventures Partners, Mack Healy & Phil Grayeski take a deep dive into a specific S-1 filing from Sana Biotechnology:


What we liked on Twitter







Events
The Collision Between Biotech And Tech - How To Navigate The Overlapping Legal Issues In This New And Emerging Landscape | Oct 12 | Boston + Virtual
Partner Kyle Faget, Co-Chair of Foley’s Health Care Practice Group will be speaking on the panel “The Collision Between Biotech And Tech - How To Navigate The Overlapping Legal Issues In This New And Emerging Landscape” of the New England Corporate Counsel Association.
Microsoft Research Summit 2022 | Oct 18-20 | Virtual
Microsoft is hosting a research summit that includes tracks focused on scientific discovery and precision medicine including a keynote by Peter Lee, Corporate Vice President for Research and Incubations at Microsoft, on the Future of Human Health, panels on Scientific Innovation and Discovery (featuring Najat Khan, PhD and Anthony Philippakis) as well as Ambient Clinical Intelligence (featuring Kenneth Harper, Yaa Kumar-Crystal, Gregory Moore MD, PhD, and Jared Pelo), and a number of talks describing cutting edge work on AI in Healthcare and the Life Sciences. Register here.
Biosample Hub Online Conference Series: Sourcing Biospecimens for Industry R&D | Oct 25 | Virtual
Part 1 of a three-part series on how to connect Biotech and Pharma companies with non-commercial Biobanks, allowing the ethical provision of reliable human biospecimens with full data provenance. Register here.
Tough Tech Summit 2022 | Oct 27-28 | Boston
The Engine’s annual summit is back! Split into two days: Invest and Build, the days are packed with pitches and talks from founders of Solugen, Sila, Mainspring, and Form Energy. Pablo will be there — say hi if you’re around!
TechBio Cocktails | Nov 9 | NYC
A few of us are organizing a happy hour for founders, operators, scientists, and investors in NYC on November 9. If you happen to be in the area, RSVP here.
Notable deals
Ginkgo acquires Altar and Circularis to provide further capabilities to customers in adaptive laboratory evolution, circular RNA, and promoter screening. Altar, a French biotechnology company that has developed a proprietary adaptive evolution platform, and Circularis, a biotechnology company with a proprietary circular RNA and promoter screening platform. Through these acquisitions, Ginkgo expects to offer new solutions to customers across multiple industries and further bolster their capabilities across the full stack of biological engineering.
Cellarity tacks on $121M series C to fund cell behaviour pipeline. The Flagship-founded company just announced its new fundraise, which brings the company’s total raised to $274M since it launched at the end of 2019. New investors include the VC wing of Korean energy company Hanwha Impact and Japanese pharma Kyowa Kirin. The company has focused on mechanisms of whole cells and their impact on disease progression. Using machine learning, Cellarity says it’s able to make digital predictions on potential targets at a faster pace than the usual screening process.
Ochre Bio raised a $30M series A from Khosla Ventures, Hermes-Epitek, Backed VC, LifeForce Capital, Selvedge, AixThera, LifeLink, EIT and angel investors from Verge Genomics, BioAge and Recursion. Employing a deep phenotyping approach, Ochre has generated spatial-sequencing, single-cell sequencing, and imaging data in over 1,000 diseased human livers across three continents. Proceeds to support the development of first candidates for IND-enabling studies, as well as expand discovery and RNA chemistry capabilities to address a wider set of serious liver-related diseases.
Field trip
The $600m business of Spirit Halloween…


The Acquired Podcast deep dives into Benchmark in a fascinating (nearly four hour) episode