BioByte 008
combatting photoshopped science, neurIPS, trends in pharma m&a and academic spinouts, challenges in mRNA therapeutics, PD-L1 for onc- ... aging?
Welcome to Decoding TechBio, a writing collective focused on the latest scientific advancements, news, and people building at the intersection of tech x bio. If you’d like to connect or collaborate, please shoot us a note here. Happy decoding!
What’s up everyone! A few of us (Patrick, Amee, Morgan) are in NYC this week. If you’re around, reach out. Also let us know if you are going to NeurIPS in New Orleans. Fill out your contact info here or feel free to reach out directly - we’d love to meet up.
What we read
Blogs
Here’s why we’re not prepared for the next wave of biotech innovation [Matthew Herper, STAT, 2022]
The last decade has seen a dizzying pace of progress across many biotechnologies and therapies. The more significant bottleneck over the next several decades will not be scientific and technological advances coming out of the lab, but will be the infrastructure for testing these therapies for translation into the clinic. Patient recruitment for clinical trials is exceedingly difficult, and the cost to run a randomized-controlled trial, the current gold standard, can cost up to hundreds of millions or billions of dollars. Herper wittily sums up the problem:
“...the U.S. health care system tends to believe that inventing brand new gadgets is the answer to everything. The result is that we try to solve problems by building faster and more expensive Ferraris when what we really need are better roads. As a result, our sports cars end up stuck in the mud.”
It is clear that more investment and better solutions and technologies for running clinical trials are necessary. Some potential solutions discussed in the article:
A system for conducting smaller, more cost-effective studies to quickly obtain early proof-points that justify further investment and larger, more expensive clinical trials
Better, faster patient recruitment for clinical trials
Simpler trials; collecting the minimum amount of data required in clinical trials to answer the question of interest
Tech-enabled platforms, such as the clinical-research platform built by Verily, for the on-demand creating of randomized trials with scalable and cheap data collection using tech like wearables
Many startups and incumbents have built platforms over the years leveraging these ideas, with varying degrees of success. What is truly needed, however, is a prioritization and reimagining of our clinical trial infrastructure, from how we incentivize clinical development, design trials, collect data, and recruit and randomize patients.
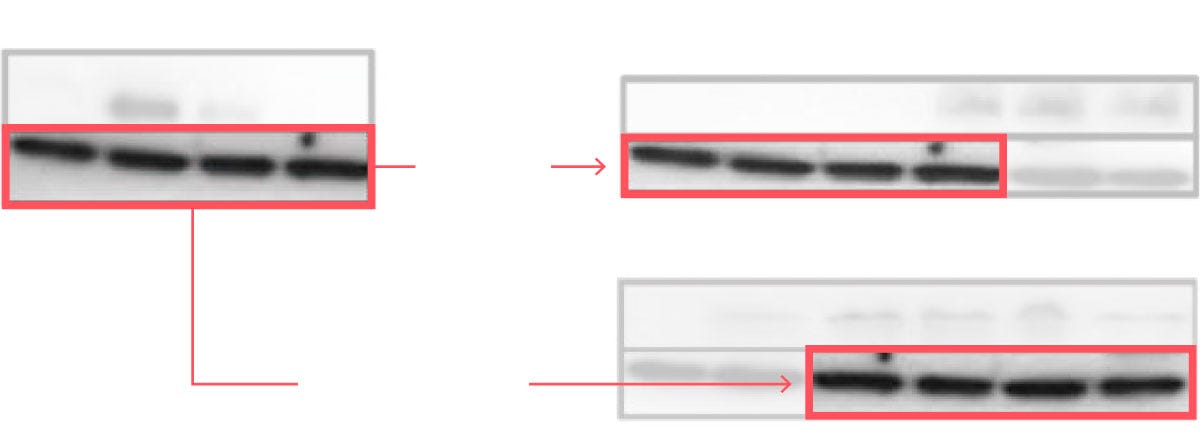
Science has a Nasty Photoshopping Problem [Dr. Elisabeth Bik, NYT Opinion, 2022]
In this opinion piece, Dr. Elizabeth Bik, microbiologist who has worked at Stanford University and for the Dutch National Institute for Health, calls for greater attention to research fraud, and specifically, photoshopped and manipulated images. Via an interactive, scrollable interface, the piece shows examples of research fraud in cell cultures, chemical structures, and microscopy images.
She also proposes a list of changes, many of which we find interesting in the context of the Open Science and decentralized publishing movements:
Journals must carry out better quality control. Publishers should hire image analysts and statistical experts to screen accepted papers before publication.
Journals need to act much faster — for example, within six months — when evidence of image manipulation arises.
We need national and international science integrity organizations that can independently investigate suspected cases of fraud and have some ability to punish the guilty.
Legitimate criticism of scientific research should receive legal protection.
Journals should pay the data detectives who find fatal errors or misconduct in published papers, similar to how tech companies pay bounties to computer security experts who find bugs in software.
Exscientia Expands Biologics Design Capability with Automated Laboratory [Exscientia, 2022]
Exscientia, one of the largest AI-first drug discovery companies focused in small molecule design, has expanded into antibody design.
The company has been focused on “shifting the curve” by improving the probability of success, time and cost involved with creating new medicines. They are now applying the same objective to biologics.
The company is using a model produced by Professor Charlotte Deane, Exscientia’s Chief Scientist of Biologics AI, which yielded accurate protein modelling up to 35,000 times faster than Alphafold2. Exscientia’s key insight is that instead of using sequence information on one of the chains of the antibody, it uses both sequences, which is more representative of its true biology.
“Our strategy is to replace current experimental discovery techniques with precision engineered de novo design of optimised, fully-human biologics. Current methods limit the discovery of new binding sites to what can be explored via animal immunisation or laboratory-based libraries. By virtually designing all aspects of a biologic in silico, we can explore a much broader target universe and create more precisely targeted therapeutics: Biologics by design not discovery, driven by AI and automated experiment, is our approach.” said Professor Charlotte Deane MBE.
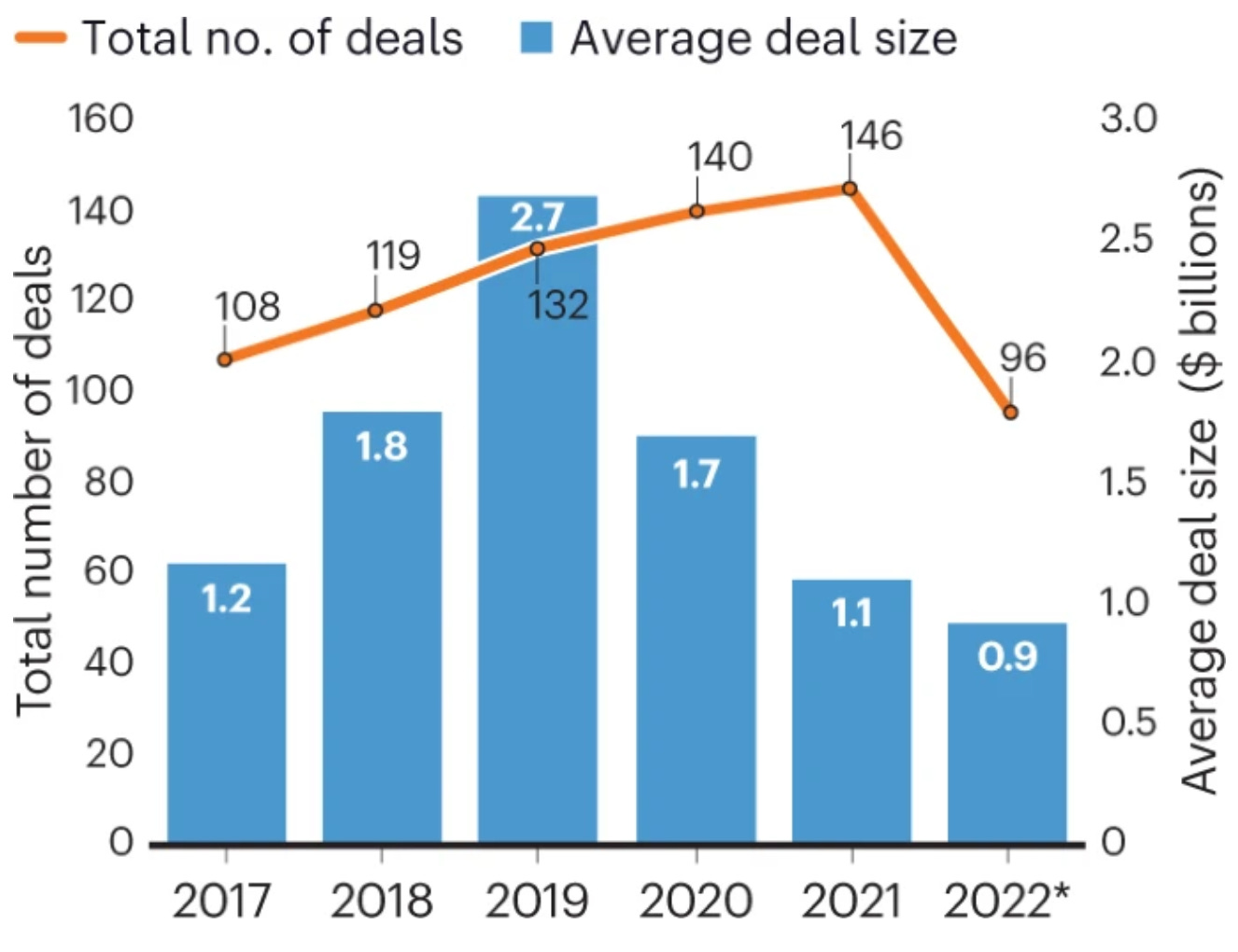
Pharma backs off biotech acquisitions [Melanie Senior, Nature Biotech, 2022]
At the start of 2022, with biotech company market caps and cash reserves in freefall, it seemed like a great opportunity for large pharmaceutical companies to acquire companies with robust pipelines, at a discount, to replenish their own.
That hasn’t happened, and instead Pharma companies have decided to sit on their cash, or partner instead.
The article is full of useful tidbits on the current financial landscape in biotech:
Good data still open the funding taps — even for public biotechs
Biotechs that aren’t quite at data readout should focus their efforts and funds on getting there — and avoid overpromising in the meantime.
Biotechs with insufficient cash to get to the next milestone have few choices. Record numbers of biotechs are exploring ‘strategic options’. Banking or advisory firms predict a more active M&A spree this autumn as a result.
Venture capital investors are preparing for the market gloom to last. They’re putting three or four years’ worth of cash into their portfolio companies, rather than one or two, to ensure they reach the next data point.
Buyers can afford to soft-pedal. Big pharma needs to do more to fill pipelines and revenue gaps. But for now, many are thinking, “Let these companies finance themselves, and we’ll pay more for them later”
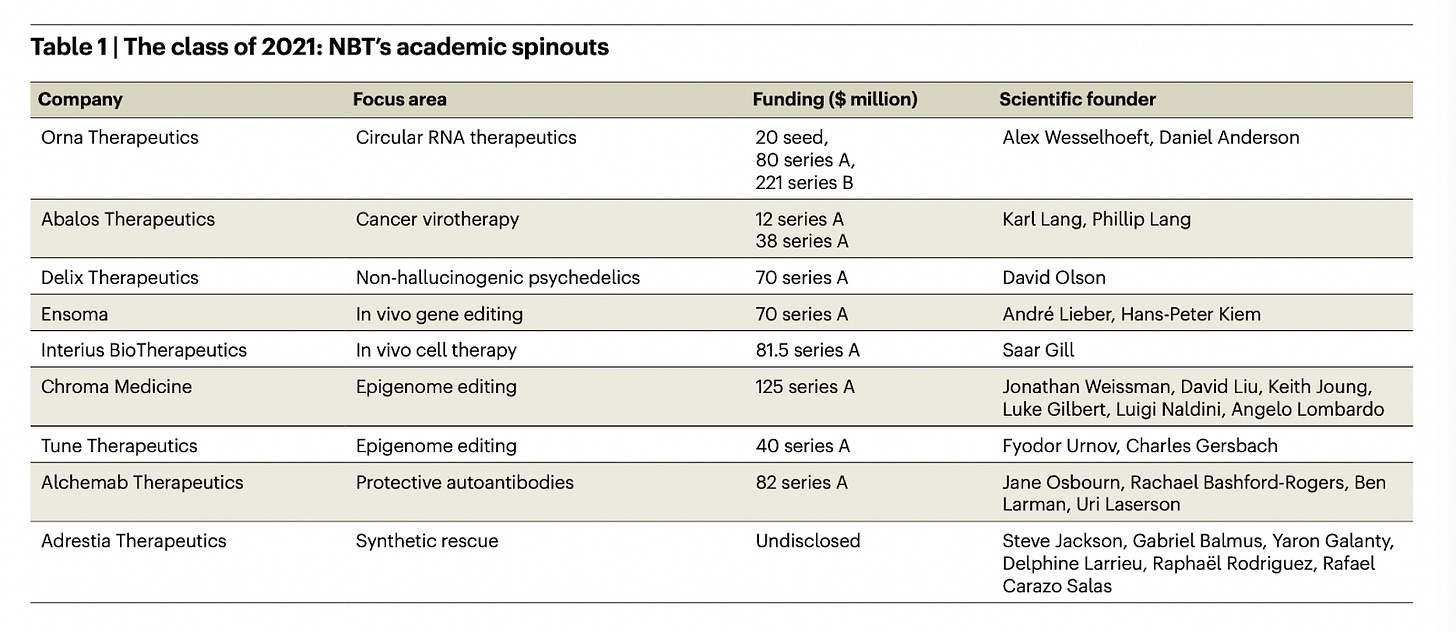
Nature Biotechnology’s academic spinouts 2021 [Eisenstein et al., Nature Biotech 2022]
As we wind down 2022, we thought it would be interesting to revisit this annual classic. Every year, Nature Biotechnology profiles startups spun out of academia – hardcore R&D and emerging technologies abound. This year, they feature companies at the forefront of epigenome editing, circular RNA therapeutics, in vivo cell and gene therapy therapy, non-hallucinogenic psychedelics, and protective autoantibodies.
Academic papers
Randomized Clinical Trials of Machine Learning Interventions in Health Care [Plana et al., JAMA Network Open, 2022]
Despite randomized-controlled trials (RCTs) being the gold standard in medicine to determine the effectiveness of an intervention, the majority of machine learning algorithms available today have not been tested in an RCT. This systematic review found a striking lack of RCTs for clinical AI. Forty-one medicine learning RCTs were identified, despite the fact there are 343 FDA-approved clinical AI interventions, a massive gap. Further, none of the reported studies adhered to the CONSORT-AI standards, widely-agreed upon standards for reporting randomized trials evaluating clinical AI interventions. A few more sobering observations from the paper:
93% of trials did not assess performance errors
90% of studies did not share code and data, making it difficult to reproduce published results
Only 49% of trials were conducted at more than 1 site, raising concerns about generalizability of results due to differences between clinical sites
Only 27% of RCTs reported participant race or ethnicity
Fortunately, the number of RCTs being conducted over time is increasing. Nonetheless, the findings highlight opportunities for improving the quality, transparency, and reproducibility of healthcare AI going forward.
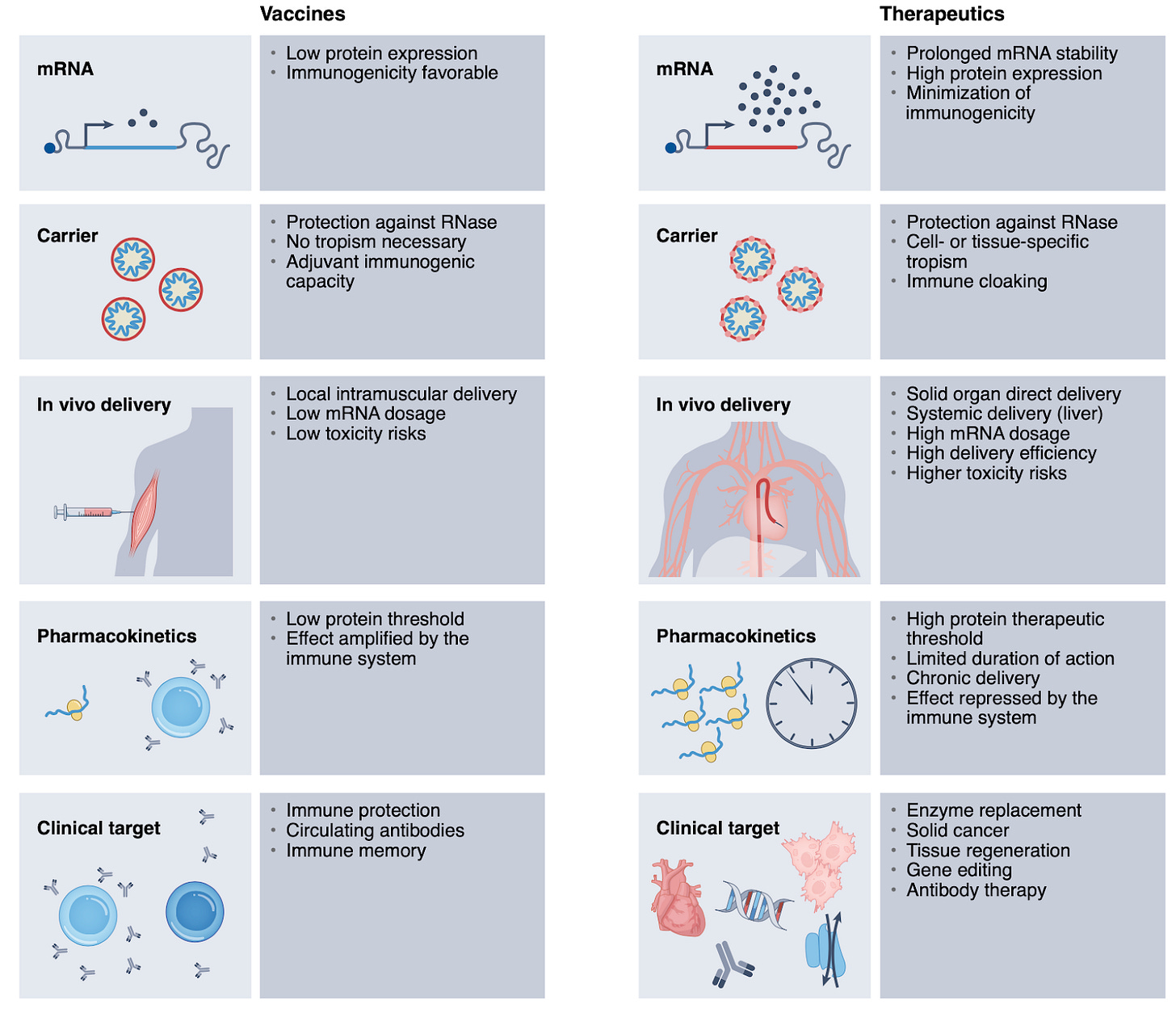
Unlocking the promise of mRNA therapeutics [Rohner et al., Nature Biotech, 2022]
The advent of the mRNA COVID-19 vaccine has led to an increased interest in its use as a therapeutic. Whilst there are clinical trials underway, there are critical factors that need to be resolved before mRNA therapeutics become widely applicable to both rare and common diseases.
The path for mRNA therapeutics presents additional technical challenges compared to those of mRNA vaccines:
High protein expression: 1000x higher protein expression to reach therapeutic threshold
Packing kinetics and dynamics: bioavailability, half-life and efficiency of lipid based carriers can be strictly rate limiting
Tissue (or cell) tropism: efficient delivery to organs aside from the liver is still challenging
Repeat dosing: for chronic disease, repeated dosing is often required, which can activate innate immunity and associated decrease in protein expression
The three sections in the review outline existing technologies developed to overcome the issues above.
Enhancing protein yield: by optimizing the mRNA cargo to minimize innate immune responses, enhance mRNA stability and maximize translation
Packing systems: advances in composition of LNPs, extracellular vesicles, cells as carriers and biomimetics
Tissue targeting: through packing system optimisation or improved drug delivery
Chronic dosing: no technological progress, but the authors suggest meticulously designed studies and detailed monitoring in small and larger animal models to help resolve most of these concerns.
Blocking PD-L1–PD-1 improves senescence surveillance and ageing phenotypes [Wang et al., Nature, 2022].
PD-L1 is a well-known target in immuno-oncology; in this paper, the authors implicate PD-L1 as a putative target for anti-ageing therapy. One of the hallmarks of aging is the accumulation of senescent cells, which leads to inflammation and age-related pathology. It turns out that senescent cells express PD-L1 heterogeneously, protecting them from T-cell surveillance. However, administering anti PD-1 antibodies to mice with normal or deranged livers reduced the population of PD-L1+ senescent cells in a CD8+T-cell dependent manner, improving a number of age-related phenotypes. Can immune checkpoint blockade be an anti-aging therapy in the near future? Only time will tell…
What we listened to
Kierstein Stead, Managing Partner at DCVC Bio, on Deep TechBio Company Creation
In this podcast, Dr. Kiersten Stead, Managing Partner at DCVC Bio, chats computational drug discovery, her investment thesis, and keys to company creation.
Pat Walters, Chief Data Officer at Relay Therapeutics, at the Broad Institute’s Machine Learning for Drug Discovery Symposium
The Broad Institute’s Machine Learning for Drug Discovery Symposium took place on Oct 24. All of the talks have been uploaded to YouTube. Check out the full playlist of videos from the symposium.
Michael Levin: Biology, Life, Aliens, Evolution, Embryogenesis & Xenobots | Lex Fridman Podcast #325
In Episode #325 of the Lex Fridman podcast, Michael Levin, biologist at Tufts University, shares more about how his lab is developing novel ways to understand and control complex pattern formation in biological systems.
Notable Deals
J.P. Morgan Launches New Life Sciences Private Capital Team Targeting Investments in Innovative Healthcare Companies. The team will focus on both early and growth stage in several therapeutic areas and technologies including AI/MLplatforms.
Concerto Biosciences Raises $23 Million in Series A to Advance Product Development, Expand Pipeline. The round led by Safar Partners and joined by Horizons Ventures and M Ventures. Concerto Biosciences designs microbial communities that restore deficient microbiomes to treat disease. Concerto’s foundational discovery platform, kChip, allows for rapid phenotypic screening of combinations of microbes to identify microbial “ensembles”—optimally performing combinations. The company’s first microbial product, “Ensemble No.2” (ENS-002), is designed to rehabilitate the skin microbiome. By taming the pathogenic behaviors of Staphylococcus aureus, ENS-002 may decrease the frequency and severity of eczema flares.
What we liked on Twitter







Events
DeSci London | Jan 15-16 2023 | The Francis Crick Institute
DeSci London will be a 2-day event focused on showcasing the potential of blockchain technology within science. Talks and workshops will cover decentralised science (DeSci) projects aiming to improve everything from publishing infrastructure to longevity, rare diseases, climate tech & SynBio.
Field Trip
Any Smino fans? We think the new album is 🔥🔥🔥
and some Taylor Swift because…balance 🙃
Did we miss anything? Would you like to contribute to Decoding TechBio by writing a guest post? Drop us a note here or chat with us on Twitter: @ameekapadia @pablolubroth @patricksmalone @morgancheatham @ketanyerneni