BioByte 009
the IRA's projected impact on biotech, systemic challenges in drug development, a creative way to drug the undruggable, the only bio reading-list you need, and more
Welcome to Decoding TechBio, a writing collective focused on the latest scientific advancements, news, and people building at the intersection of tech x bio. If you’d like to connect or collaborate, please shoot us a note here. Happy decoding!
Happy Tuesday! Thanks to everyone who came out to the TechBio happy hour in NYC last week. We had a blast meeting so many of you and are excited for more events, especially as JPM season rolls around. On that note, let us know if you’re going to NeurIPS in New Orleans. We’d love to meet up.
This past week has felt overwhelming in so many ways and while it may seem like the bio community has been relatively insulated, think again. That being said, we’ll leave the FTX coverage to Matt Levine and stick to our bio roots. Let’s get into it.
What we read
Blogs
You Have Chosen … Poorly: Why Drug Developers Make Bad Decisions [David Shaywitz, Timmerman Report, 2022]
Sharp analysis in the Timmerman Report by David Shaywitz analyzing the reasons for the low odds of success in drug development. Two primary factors were discussed, and the piece concludes with a discussion of the role of AI.
Remove organizational bias: David Grainger (the Daniel Kahneman of biopharma?) argues that drug developers make poor decisions not because of the quality of data available to them, but to biases like infrastructure, narrative, and commercial bias. Infrastructure bias (e.g., employees, lab space) results from inertia around therapeutic programs that make it difficult to shut down fledgling campaigns. The story a biotech tells about themselves to investors, for example about a focus on a specific technology, tends to become ingrained as narrative bias. Commercial motivations bias R&D towards specific indications. Grainger’s proposal is to instead develop drugs with a laser-like focus on specific assets, rather than broad TAs, enabling-technologies, etc to avoid the above biases.
Improve translational models: consistent readers of DTB will know we are fans of Jack Scannell. We’ve covered Jack’s recent Nature Reviews Drug Discovery paper in a previous edition, but to reiterate: Jack’s thesis is that the most important factor in drug development is predictive validity, or how well a preclinical or translational model predicts success in clinical trials. Proposed solutions for drug hunters? Spend more time evaluating and improving models, focus first on diseases that we can feasibly model, and thoughtfully combine complementary pre-clinical models. The financial incentives for many of these solutions are weak, so a consortium of biopharma organizations focused on developing better translational models may be needed.
According to the above frameworks, what role will AI play in improving the low probability of success in clinical trials? Probably not much. A big part of the problem is that not enough AI is focused on the most important problems in drug development that account for low PoS, namely predicting safety and efficacy and late-stage clinical trials. For this, we need better data translational models, better systems for relating data collected in late-stage trials to the discovery phases of drug development, and better infrastructure and systems for delivering data and insights to key decision makers to minimize bias. A better ELN won’t solve your problems [Jesse Johnson, Scaling Biotech, 2022]
Jesse Johnson argues that the lack of shared mental models between wet and dry lab teams is one of the biggest problems facing techbio companies. In this article, he describes how Electronic Lab Notebooks (ELNs) and Laboratory Information Management System (LIMS) are designed to solve problems that were big 10 to 20 years ago.
‘The tipping point is coming’: Unprecedented exodus of young life scientists is shaking up academia [Jonathan Wosen, STAT News, 2022]
“The tipping point is coming,” Eastmond said. “There are a lot of conversations happening about how we do this moving forward, because I don’t think it is sustainable the way that it’s being done currently.”
It is probably of no surprise to the Decoding TechBio readers that there has been a steady increase of PhDs heading to industry rather than staying in academia. Wosen describes how the low salaries and intense workloads without the likely promise of a tenure has led to many biomedical PhDs into biotech companies. This has created a vacuum in academia where many researchers are unable to hire postdocs for their labs. This tipping point, however, might be the push that the academic life science enterprise requires in order to become more competitive with industry.
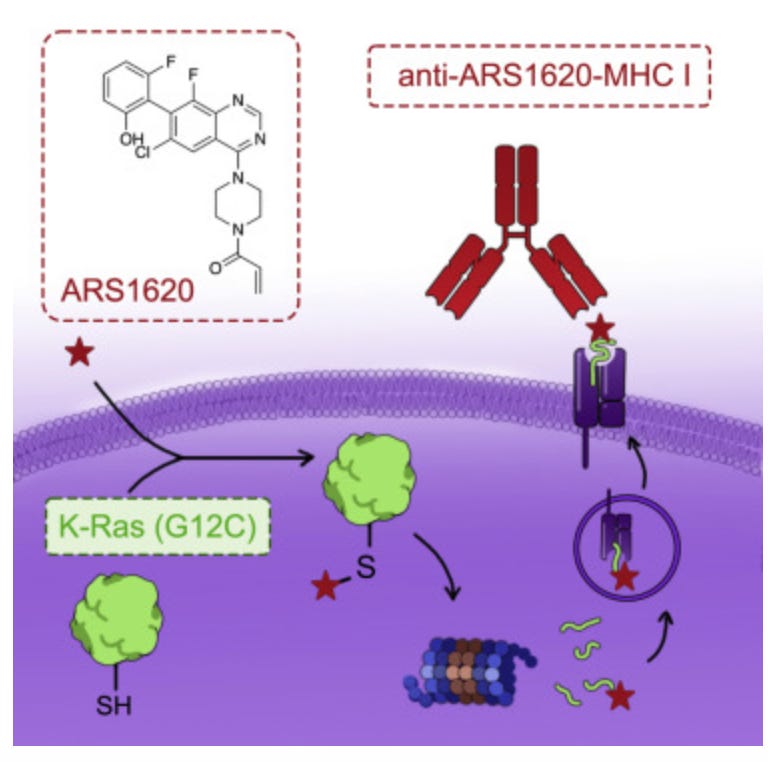
Axial Discovery – Immunotherapies and the Undruggable Genome [Joshua Elkington, 2022]
Josh breaks down some great science, as always. Here, he dives into recent work out of UCSF (Kevan Shokat, Charles Craik, Ziyang Zhang). The authors develop a novel approach to immunotargeting mutant K-RAS G12C. Briefly, they found that certain covalent K-RAS inhibitors acted as haptens, resulting in their presentation as neoantigens by MHC-I. They subsequently were able to develop bispecific T-cell engagers that elicited a cytotoxic T-cell response against K-RAS G12C cells, including those resistant to direct inhibition. Why does this all mean? Essentially, this study shows that we can use mutated proteins as an immunotherapy, dodging the need to create novel chemistries. Josh explains this well:
Recent work out of UCSF has figured out a way to guide the immune system to target cancer cells with certain mutated versions of proteins currently considered undruggable. Versus directly drugging these proteins, an alternative route is developing an immunotherapy to go after them and circumvent the challenges of developing chemical matter that need to directly engage these targets and are also drug-like.
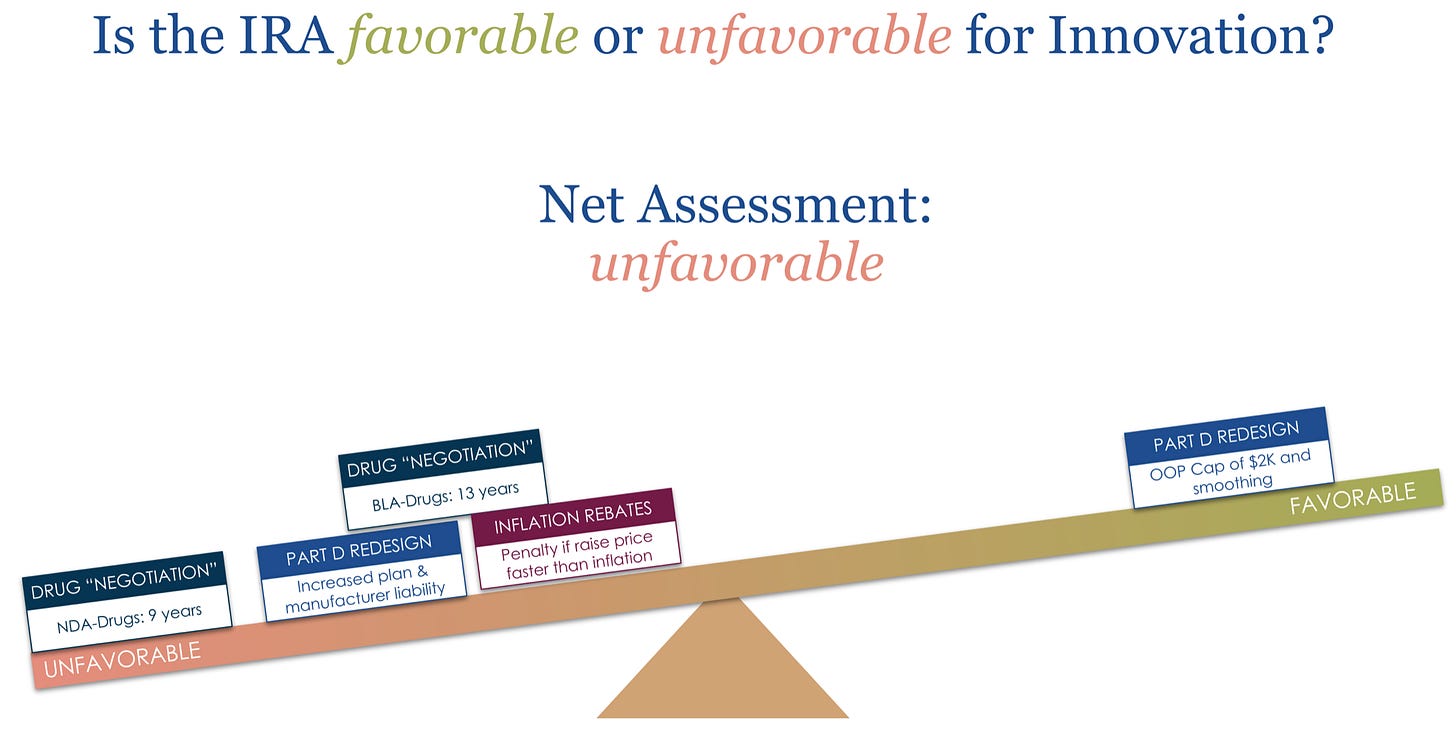
Impact of the Inflation Reduction Act for Biotech Builders… and (how) can it be fixed? [No Patient Left Behind and RA Capital, September 2022]
This 160-slide presentation may not need an introduction… In this presentation and companion webinar, Peter Kolchinksy and the team at No Patient Left Behind articulate the game-theoretic and economic implications of the Inflation Reduction Act (IRA) for burgeoning biotech companies and discuss both its favorability and unfavorability for innovation.
Spoiler: they deemed it net unfavorable. It will be interesting to see how many biotech-specific exceptions may be carved out accordingly.
Academic papers
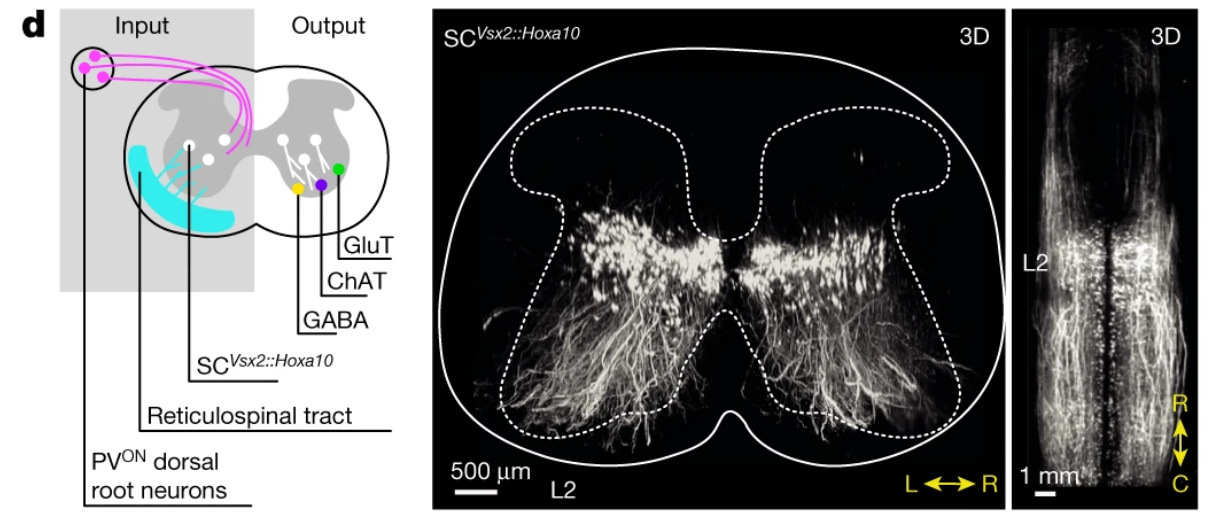
The neurons that restore walking after paralysis [Kathe et al., Nature, 2022]
Why it matters: Taken together, these elegant experiments identify a small subpopulation of excitatory neurons in the lumbar spinal cord that are both necessary and sufficient for EES-mediated recovery after SCI. Neuroscientists now have a map of neuronal subtypes and genes that will aid the search for targeted, circuit-based treatments of SCI. Epidural (i.e., on top of the protective covering of the spinal cord known as the dura) electrical stimulation (EES) is an emerging treatment for spinal cord injury (SCI), but the mechanism by which EES restores walking is poorly understood. Grégoire Courtine’s lab at the Swiss Federal Institute of Technology designed a series of human and animal experiments to generate high-resolution cell subtype and molecular maps that account for EES-mediated rehabilitation after SCI.
First, the authors showed that EES combined with a body-weight support system enabling overground walking (figure below) restored walking ability to varying degrees in a group of 9 SCI patients. A key question in the field has been how EES treatment induces reorganization of neural circuits. To measure neuronal plasticity and remodeling that accounts for recovery after SCI, the metabolic activity of the spinal cord was measured before and after EES using positron emission tomography (PET). Interestingly, metabolic activity actually decreased after rehab with EES, suggesting motor recovery results from spatially precise (re)activation of neuronal subpopulations.
To identify the cell subtypes and genetic signatures associated with motor recovery after SCI, the authors turned to a mouse model. Mice were outfitted with a similar EES device and rehab protocol after SCI, and after replicating the findings observed in the human experiments, single-nucleus RNA sequencing and spatial transcriptomics were used to characterize the molecular signature of motor recovery. A specific type of excitatory neuron (V2a) in the lumbar spinal cord was identified that preferentially responds to EES. Silencing V2a neurons prevented EES-mediated recovery of walking, and activating these neurons produced improvements in walking.
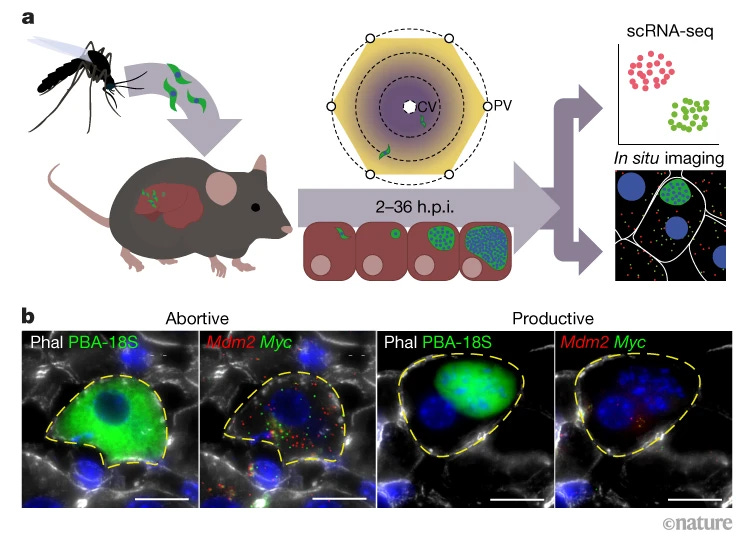
A spatiotemporally resolved single-cell atlas of the Plasmodium liver stage [Afriat et al., Nature, 2022]
Why it matters: Malaria, caused by the parasite Plasmodium, is one of the deadliest infectious diseases in the world: in 2020, there were an estimated >240 million cases of malaria worldwide, and over 600 thousand deaths. The life cycle of the parasite is extremely complex, with a variety of stages and mechanisms to avoid the immune system. In this paper, Afriat et al., decode the molecular underpinnings of the liver stage of Plasmodium at a high spatial resolution, providing insights that may be harnessed for future therapeutic or preventive efforts.The authors infected mice with GFP-expressing Plasmodium, sorted infected hepatocytes, and performed scRNA-seq at various time points post-infection. This allowed them to assess gene expression over time in both parasites and host hepatocytes in different zones of the hepatocellular cords. They unearthed a number of interesting findings: for example, infected hepatocytes increased their expression of a number of stress-response genes, iron sequestration genes (parasites use iron for critical metabolic processes), and decreased fatty-acid synthesis genes (perhaps to starve the parasites of critical lipids). In reciprocation, the parasites upregulated their own fatty acid synthesis genes. Additionally, the authors identified an anatomy-dependent nature of parasite maturation: they matured quicker in the inner zones as compared to the outer zones.
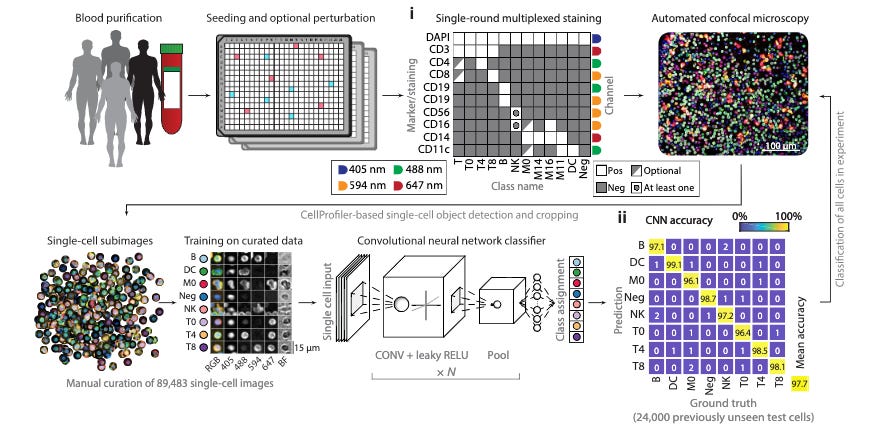
Multiplexed high-throughput immune cell imaging reveals molecular health-associated phenotypes [Severin et al., Science Advances]
What is the link between immune cell phenotypes and our molecular and physical health? This paper analyzed various immune cell phenotypes within human cohorts to understand correlations with key donor attributes such as age, gender, and blood pressure. Leveraging multiplexed immunofluorescence, automated microscopy, and convolutional neural networks applied to serum samples, Severin et al. clustered and described eight immune cell classes: T cell subsets (CD4+, CD*+, and CD4-CD8-), monocytes, dendritic cells, natural killer cells, B cells, and nucleated immune cells, which were then mapped to the following phenotypic information: age, gender, blood pressure, and inflammatory state with various immune cell phenotypes.
Our excitement around this work is two-fold: the methodology allowed for an appreciation of heterogeneity between individual donors as well as that observed within cells of the same class for a given donor. Arguably the phenotypes explored in the study (age, gender, blood pressure, and inflammatory state) are likely a small segment of the total phenotypic space correlated with the immune cell states observed in the donor population.
What we listened to
Friend of the community Guillermo Vela, CEO and Co-Founder of NeuScience, was interviewed on Musty’s Big Picture Medicine podcast on Guillermo’s recent article on TechBio, his contrarian view of cancer, why AI will not make drug discovery faster, better, or cheaper, and more. An overall refreshing perspective from a TechBio supporter and skeptic.
Logan Bartlett interviews Peter Fenton, legendary tech VC from Benchmark. Biggest take away from Peter: stay humble, stay hungry, and don’t have an ego no matter how successful you are.
Friend of DTB, Nitya Sridhar, interviews Ginkgo Bioworks’ co-founder Reshma Shetty on her podcast New Frontiers. They cover new policy decisions in biomanufacturing, where synthetic biology is headed, and the origin story of Ginkgo in a fun 30-min chat. Perfect for your commute home.
Atlas Venture partner and LifeSciVC author Bruce Booth shares his Year in Review for 2022.
Notable Deals
Sequencing.com raised $5M from Lerer Hippeau, Red Sea Ventures, Red Antler, Gaingels, Global Founders Capital, and others. The company, founded in 2016, provides a direct-to-consumer marketplace for DNA analysis, management, and storage. They expect to add whole genome sequencing capabilities with this round.
Juvena raised a $41M Series A from Mubadala, Horizons, and Bison Ventures for their platform seeking to convert secreted proteins into drugs for rejuvenation and aging.
Sensorium Therapeutics raised a $30M round from Santé Ventures, Route 66, and others for their platform focused on developing drugs from plant-derived psychoactive compounds.
WellTheory raised a $7.2M Seed round led by Accel with participation from Lux Capital, Box Group, and Coalition. Their platform helps manage lifestyle-interventions for autoimmune disease.
What we liked on Twitter





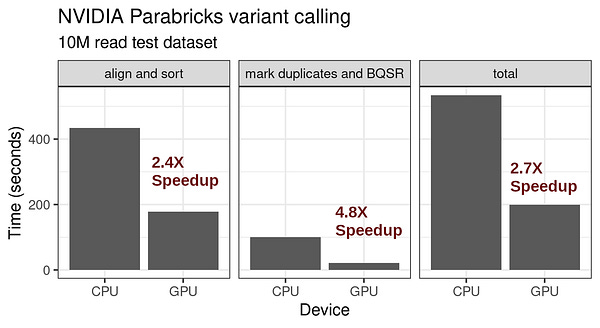





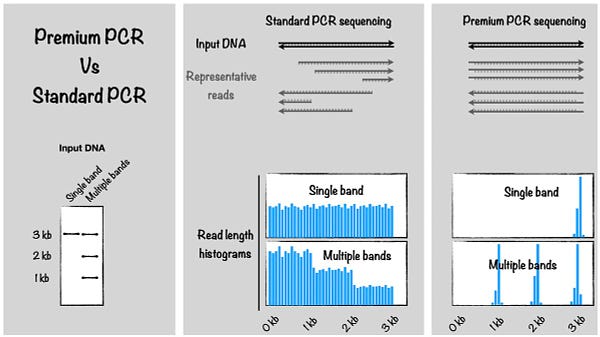

Events
JP Morgan Healthcare Conference | January 9-12 | San Francisco
It’s never too early to start prepping for JP Morgan. We’re expecting this years to be one of the biggest given the last three were virtual-only. Stay tuned for a guide on specific events, but start making those travel plans!
Field Trip

It’s that time of year again…..bust out your cooking chops and learn how to prep a fine turkey, sooner than later. Sending this next week would be too late!
Did we miss anything? Would you like to contribute to Decoding TechBio by writing a guest post? Drop us a note at here or chat with us on Twitter: @ameekapadia @pablolubroth @patricksmalone @morgancheatham @ketanyerneni







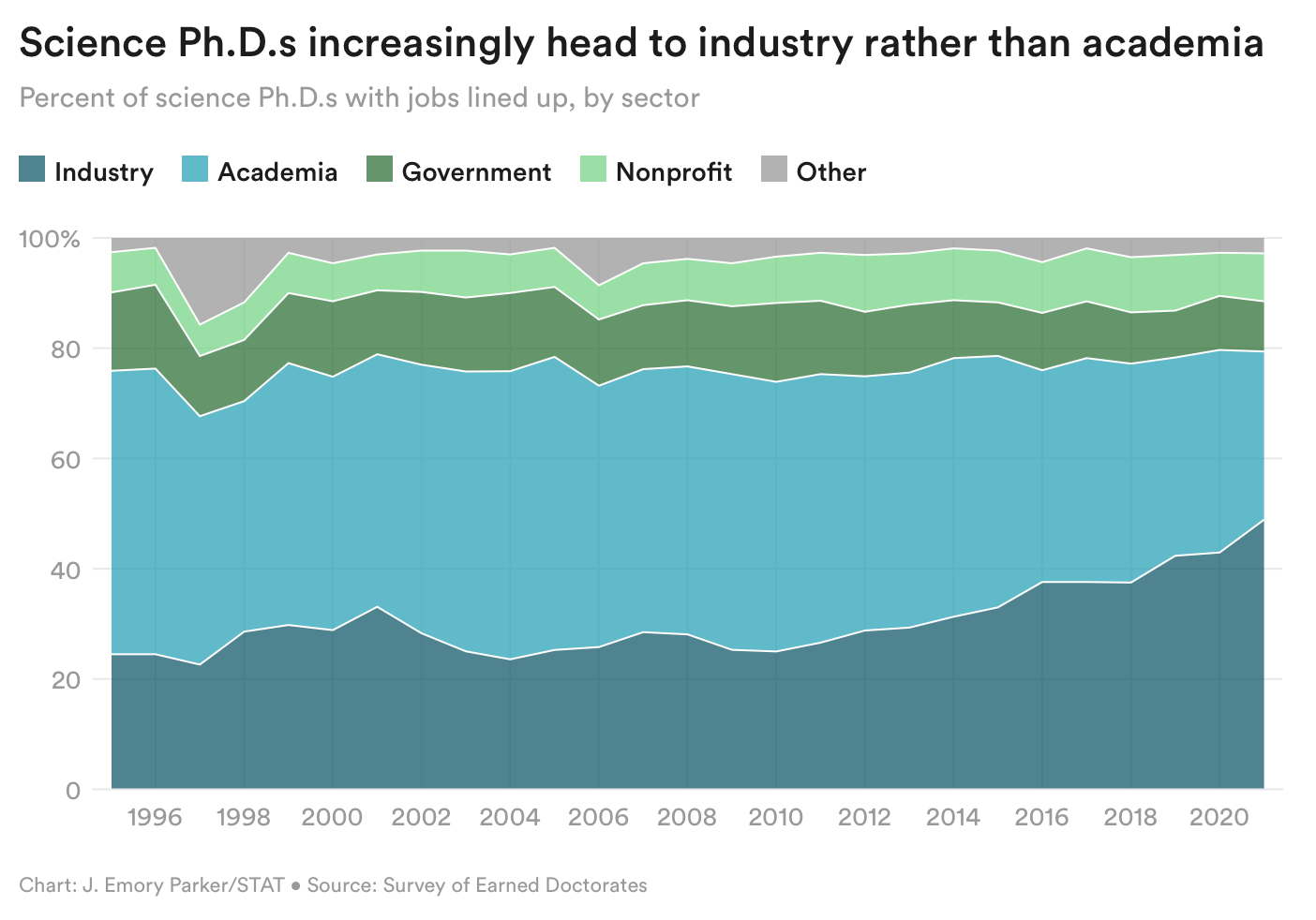




You guys should start a magazine! Love it!!